Key Points
Btk inhibitors specifically block platelet thrombus formation on atherosclerotic plaque but spare physiologic hemostasis.
Irreversible Btk inactivation in platelets incapable of enzyme resynthesis allows low intermittent drug dosing for antiatherothrombosis.
Abstract
Interaction of von Willebrand factor (VWF) with platelet glycoprotein Ib (GPIb) and interaction of collagen with GPVI are essential for thrombus formation on ruptured or eroded atherosclerotic plaques (atherothrombosis). GPIb and GPVI signal through Bruton tyrosine kinase (Btk), which can be blocked irreversibly by oral application of ibrutinib, an established therapy for chronic lymphocytic leukemia (CLL) with long-term safety. We found that ibrutinib and the novel Btk inhibitors acalabrutinib and ONO/GS-4059 block GPVI-dependent static platelet aggregation in blood exposed to human plaque homogenate and collagen but not to ADP or arachidonic acid. Moreover, Btk inhibitors prevented platelet thrombus formation on human atherosclerotic plaque homogenate and plaque tissue sections from arterially flowing blood, whereas integrin α2β1 and VWF-dependent platelet adhesion to collagen, which is important for physiologic hemostasis, was not affected. This plaque-selective platelet inhibition was also observed in CLL patients taking 450 mg of ibrutinib and in volunteers after much lower and intermittent dosing of the drug. We conclude that Btk inhibitors, by targeting GPIb and GPVI signal transduction, suppress platelet thrombus accretion from flowing blood on atherosclerotic plaque but spare hemostatic platelet function. Btk inhibitors hold promise as the first culprit lesion–focused oral antiplatelet drugs and are effective at low doses.
Introduction
Erosion or rupture of atherosclerotic plaques exposes material that arrests circulating platelets, triggers intraluminal thrombosis, and can precipitate myocardial infarction or stroke. Collagens accumulating in atherosclerotic plaques differ structurally from collagens of healthy connective tissue that may alter their platelet reactivity.1-3 Decisive thrombogenic plaque components are morphologically diverse collagen types I and III fibers that induce platelet aggregation under static and flow conditions via glycoprotein VI (GPVI),2,4,5 which, in addition to the integrin α2β1, is a main collagen receptor on platelets.6-9
To further improve antiplatelet therapy with aspirin (acetylsalicylic acid [ASA]) and/or a P2Y12 antagonist without intolerably increasing bleeding risk, compounds that specifically target plaque-triggered platelet activation with high efficacy, but that leave physiologic hemostasis intact, would be highly desirable. Targeting von Willebrand factor (VWF) and platelet GPIb and GPVI might offer such a strategy.10 Anti-VWF and GPVI-competing agents (recombinant GPVI-Fc) underwent clinical phase 3 testing,10 and anti-GPVI antibodies inhibited thrombosis triggered by plaque injury in murine models.11,12 Of note, antibodies against GPVI or the VWF binding site of platelet GPIb reduced human plaque–induced platelet thrombus formation from flowing blood more efficiently than did ASA and P2Y12 antagonists.13,14 Importantly, anti-GPVI antibodies blocked plaque-induced platelet thrombus formation but left integrin α2β1–dependent platelet activation by collagen, which is important in physiologic hemostasis, untouched.2,5,15
An oral small molecule approach might be offered by inhibitors of Bruton tyrosine kinase (Btk), which appears to be critical for VWF/GPIb- and collagen/GPVI-triggered platelet signal transduction.16-18 Upon platelet GPIb engagement with VWF or GPVI stimulation by collagen, the tyrosine kinases Lyn and Syk are activated first and initiate the assembly of a signaling complex of adapter proteins and enzymes, including Btk and PLCγ2.16,19 Btk is phosphorylated by Syk and Lyn, further autophosphorylated, and then participates in the tyrosine phosphorylation and activation of the effector protein PLCγ2.19 This increases cytosolic Ca2+ and activates protein kinase C, the 2 main downstream signals for platelet activation.20
Ibrutinib (PCI-32765), an orally active Btk inhibitor, has been approved for the treatment of chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). Ibrutinib binds covalently to cysteine 481 in the active site, thus irreversibly inhibiting Btk.21 Novel and even more selective Btk inhibitors have been developed, and some (acalabrutinib, ONO/GS-4059, and BGB-3111) have passed phase 3 trials in relapsed or refractory B-lymphoid malignancies without major bleeding events.22-24
We hypothesized that Btk inhibition downstream of GPVI might selectively prevent plaque-triggered, but not native collagen–triggered, platelet thrombus formation under arterial flow, because, in normal hemostasis, loss of GPVI function is compensated for, in part, by the other major platelet collagen receptor, integrin α2β1.15,25 However, the integrin α2β1 is not involved in platelet thrombus formation on plaque collagen, which is mediated by GPVI.2,5 This may be explained by the differences in collagen types I and III structures between atherosclerotic plaques and healthy connective tissues.1-3,5 Therefore, we explored the potential of Btk inhibitors to specifically prevent atherosclerotic plaque–triggered platelet aggregation in vitro and ex vivo in blood from patients on ibrutinib and human volunteers after ibrutinib intake.
Materials and methods
For details, please see supplemental Methods, available on the Blood Web site.
Declaration of Helsinki
Informed consent was obtained from patients and healthy volunteers, as approved by the Ethics Committee of the Faculty of Medicine of the University of Munich, and in accordance with the ethical principles for medical research involving human subjects, as set out in the Declaration of Helsinki.
Human carotid atherosclerotic plaques
Atherosclerotic tissue specimens were donated from patients who underwent endarterectomy for high-grade carotid artery stenosis.2,4,26 The atheromatous plaques were carefully dissected under sterile conditions from other regions of the atherosclerotic tissue.2,4,26 The plaques were processed to obtain homogenates or 3-µm cryosections. Plaque homogenates from 5 patients were pooled.
Blood collection for in vitro and ex vivo studies
For experiments on Btk inhibitors in vitro, blood was obtained from healthy adults who had not taken any platelet inhibitor for ≥2 weeks. Venous blood was drawn into a syringe containing recombinant hirudin (final concentration in blood ∼200 U/mL; 13 µg/mL).
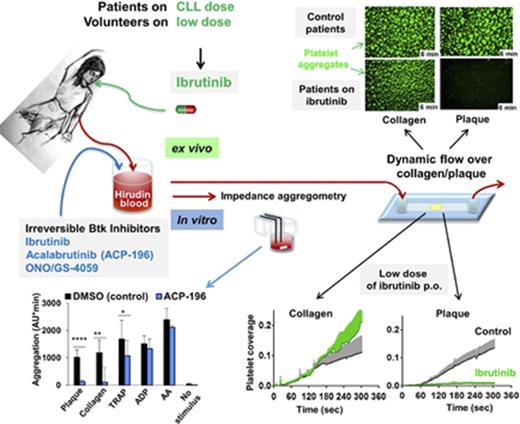
For ex vivo experiments, CLL patients (n = 5) on continuous ibrutinib treatment (IMBRUVICA; 3 × 140-mg capsules daily; Janssen Pharmaceutica NV, Beerse, Belgium) and control patients (n = 5) with platelet counts >150 × 109/L were asked to participate to allow analysis of platelet function in blood unimpaired by its platelet concentration. Four of the control patients had CLL, and 1 had MCL; they were not in need of treatment at this time. For each experimental day, a pair consisting of 1 ibrutinib patient and 1 control patient with similar platelet counts was matched, and their platelet functions were tested in parallel.
To test ibrutinib as possible antiplatelet therapy, 2 healthy male physicians (61 and 66 years old) took a loading dose of IMBRUVICA (3 × 140 mg), followed by a low dose (140 mg/d) for 1 week (donor A) or a very low dose (140 mg every other day) for 1 week (donor B).
Platelet aggregation in blood
Platelet thrombus formation in flowing blood
For experiments in flowing blood, glass cover slips coated with fibrillar Horm collagen (20 µg/mL), soluble collagen (100 µg/mL), plaque homogenates, or plaque tissue sections were assembled into parallel plate flow chambers. Blood was incubated with 3,3′-dihexyloxacarbocyanine iodide (DiOC6; 1 µM) for fluorescence labeling of platelets. The flow chambers were perfused with blood (shear rates 600/s and 1500/s), after incubation with dimethyl sulfoxide (DMSO) 0.1% (control), ibrutinib, acalabrutinib, or ONO/GS-4059 for 15 minutes at 37°C, or with blood from patients on ibrutinib therapy or volunteers on low-dose ibrutinib.
In vitro closure time
Statistics
Values are given as the mean ± standard deviation (SD) of n experiments. After confirmation of normality, means of 2 parallel experimental conditions were compared using a paired Student t test (*P < .05, **P < .01, ***P < .001), more than 2 concurrent experimental conditions were tested by ANOVA for repeated measures, followed by pair comparisons by the Bonferroni method using the SigmaStat package. If a normal distribution was not assured, corresponding nonparametric tests were applied (significance is indicated by § in supplemental Figure 10).
Results
Ibrutinib, acalabrutinib, and ONO/GS-4059 inhibit plaque- and collagen-induced platelet aggregation in blood under static conditions
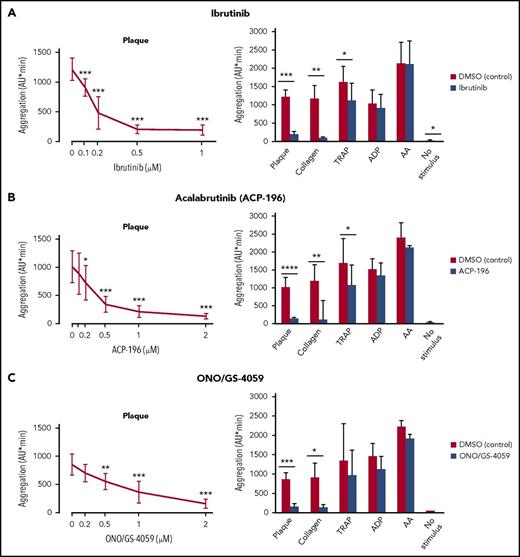
Ibrutinib (1 µM) blocked (>90%) static platelet aggregation upon stimulation with plaque and collagen fibers (Figure 1A). Ibrutinib showed similar dose-response curves for preventing aggregation by plaque or collagen, with 50% inhibition concentration (IC50) values of 0.18 ± 0.05 µM (Figure 1A, left panel), and 0.12 ± 0.04 µM (data not shown), respectively. Maximal suppression was reached at 0.5 µM (Figure 1A, left panel). Ibrutinib also blocked ristocetin-induced platelet aggregation, which is dependent on VWF binding to platelet GPIb.14 The IC50 value was 0.085 ± 0.017 µM, and maximal suppression was reached at 0.5 µM (supplemental Figure 1). In contrast, ibrutinib reduced aggregation stimulated by TRAP only moderately (−31%). Ibrutinib suppression of platelet aggregation stimulated by plaque and collagen was maintained over the entire 10-minute observation period (supplemental Figure 2A). Platelet aggregation by adenosine diphosphate (ADP), arachidonic acid (AA), and PAR-4 stimulation was only marginally affected (−13%, −1.5%, and −15%, respectively; Figure 1A; supplemental Figure 3).
Effects of ibrutinib, acalabrutinib (ACP-196), and ONO/GS-4059 on static platelet aggregation in blood stimulated by plaque, collagen, TRAP, ADP and AA. Blood samples were incubated for 15 minutes with solvent (DMSO, 0.1%) or Btk inhibitors before stimulation for 10 minutes with plaque (833 µg/mL), collagen (0.1-0.3 µg/mL), TRAP (5 µM), ADP (5µM), AA (0.6 mM), or buffer (no stimulus). Left panels: dose-response curves of ibrutinib (A), acalabrutinib (ACP-196) (B), and ONO/GS-4059 (C) on plaque-induced platelet aggregation. Right panels: effects of ibrutinib (1 µM) (A), ACP-196 (2 µM) (B), and ONO/GS-4059 (2 µM) (C) on plaque-, collagen-, TRAP-, ADP-, and AA-induced platelet aggregation and spontaneous platelet aggregation (no stimulus). Data are mean ± SD (n = 5). *P < .05, **P < .01, ***P < .001, ****P < .0001.
Effects of ibrutinib, acalabrutinib (ACP-196), and ONO/GS-4059 on static platelet aggregation in blood stimulated by plaque, collagen, TRAP, ADP and AA. Blood samples were incubated for 15 minutes with solvent (DMSO, 0.1%) or Btk inhibitors before stimulation for 10 minutes with plaque (833 µg/mL), collagen (0.1-0.3 µg/mL), TRAP (5 µM), ADP (5µM), AA (0.6 mM), or buffer (no stimulus). Left panels: dose-response curves of ibrutinib (A), acalabrutinib (ACP-196) (B), and ONO/GS-4059 (C) on plaque-induced platelet aggregation. Right panels: effects of ibrutinib (1 µM) (A), ACP-196 (2 µM) (B), and ONO/GS-4059 (2 µM) (C) on plaque-, collagen-, TRAP-, ADP-, and AA-induced platelet aggregation and spontaneous platelet aggregation (no stimulus). Data are mean ± SD (n = 5). *P < .05, **P < .01, ***P < .001, ****P < .0001.
Also, acalabrutinib and ONO/GS-4059 dose-dependently inhibited platelet aggregation by plaque (up to −87% and −83%, respectively), with IC50 values of 0.34 ± 0.19 µM and 0.79 ± 0.33 µM, respectively (Figure 1B-C, left panels). These novel second-generation Btk inhibitors also inhibited platelet aggregation induced by collagen (−89% and −85%, respectively), but they affected aggregation by TRAP (−30% and −27%, respectively) much less and only marginally inhibited aggregation by ADP, AA, or PAR-4 stimulation (Figure 1B-C, right panels; supplemental Figure 3). Acalabrutinib and ONO/GS-4059 suppression of platelet aggregation stimulated by plaque or collagen was maintained over the entire observation time of 10 minutes (supplemental Figure 2B-C).
Sufficient GPCR signaling could overcome inhibition of plaque stimulation by Btk inhibitors. ADP added to samples containing Btk inhibitors and plaque could bypass the effect of Btk inhibitors on plaque (supplemental Figure 4).
To support that plaque selectivity is specific for Btk inhibitors, the Src family kinase inhibitor PD173952 was tested. This inhibitor dose-dependently inhibited platelet aggregation by plaque and collagen, as well as by TRAP and ADP (supplemental Figure 5).
Ibrutinib, acalabrutinib, and ONO/GS-4059 inhibit platelet thrombus formation under arterial flow conditions stimulated by plaque but not by collagen fibers
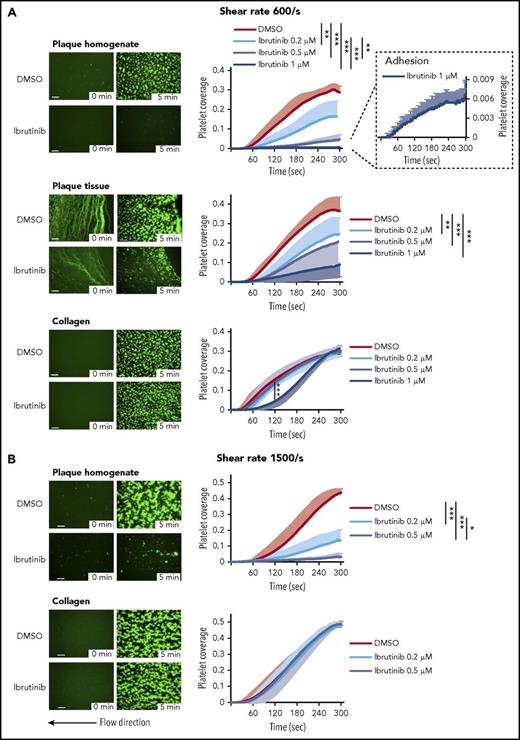
To simulate platelet activation by plaque rupture, human blood was perfused through a parallel plate flow chamber over coverslips coated with human plaque homogenate or plaque tissue sections at physiological wall shear rates of healthy carotid and coronary arteries (600/s) and at rates over mildly stenotic coronary lesions (1500/s).
Ibrutinib (1 µM) abolished platelet aggregation onto plaque homogenate (−98 ± 1%), but platelet adhesion was still observed (Figure 2A). It also strongly inhibited platelet thrombus formation on plaque tissue sections (−76 ± 16%) but only delayed full aggregate formation (8 ± 2%) on native collagen fibers (Figure 2A). Highly significant effects of ibrutinib were detectable at least down to 0.2 µM (Figure 2A). DMSO solvent did not affect the platelet responses to plaque and collagen (supplemental Figure 6).
Ibrutinib inhibits plaque-induced, but not collagen-induced, platelet aggregate formation under arterial flow at shear rates of 600/s and 1500/s. Blood samples were incubated with DiOC6 for platelet labeling and with solvent control or ibrutinib for 15 minutes. (A) Perfusion at 600/s (calculated shear stress 19 dyn/cm2). Representative micrographs show the effect of DMSO (0.1%) or ibrutinib (1 µM) on platelet deposition onto plaque homogenate, plaque tissue sections, and collagen at 0 and 5 minutes after the start of blood flow (left panels). They also show the formation of platelet aggregates in controls and their inhibition by ibrutinib. Effect of ibrutinib on the kinetics of plaque- and collagen-induced platelet deposition (right panels). Residual plaque-induced platelet deposition with 1 µM ibrutinib is shown at an enlarged scale. (B) Perfusion at 1500/s (calculated shear stress 48 dyn/cm2). Representative micrographs show the effect of DMSO (0.1%) or ibrutinib (0.5 µM) on platelet deposition onto plaque homogenate and collagen at 0 and 5 minutes after the start of blood flow (left panels). Effects of ibrutinib (0.2 and 0.5 µM) on the kinetics of plaque- and collagen-induced platelet deposition (right panels). Data are mean ± SD (n = 5). Statistical significance at 5 minutes after the start of blood flow is indicated. Scale bars = 100 μm. *P < .05, **P < .01, ***P < .001.
Ibrutinib inhibits plaque-induced, but not collagen-induced, platelet aggregate formation under arterial flow at shear rates of 600/s and 1500/s. Blood samples were incubated with DiOC6 for platelet labeling and with solvent control or ibrutinib for 15 minutes. (A) Perfusion at 600/s (calculated shear stress 19 dyn/cm2). Representative micrographs show the effect of DMSO (0.1%) or ibrutinib (1 µM) on platelet deposition onto plaque homogenate, plaque tissue sections, and collagen at 0 and 5 minutes after the start of blood flow (left panels). They also show the formation of platelet aggregates in controls and their inhibition by ibrutinib. Effect of ibrutinib on the kinetics of plaque- and collagen-induced platelet deposition (right panels). Residual plaque-induced platelet deposition with 1 µM ibrutinib is shown at an enlarged scale. (B) Perfusion at 1500/s (calculated shear stress 48 dyn/cm2). Representative micrographs show the effect of DMSO (0.1%) or ibrutinib (0.5 µM) on platelet deposition onto plaque homogenate and collagen at 0 and 5 minutes after the start of blood flow (left panels). Effects of ibrutinib (0.2 and 0.5 µM) on the kinetics of plaque- and collagen-induced platelet deposition (right panels). Data are mean ± SD (n = 5). Statistical significance at 5 minutes after the start of blood flow is indicated. Scale bars = 100 μm. *P < .05, **P < .01, ***P < .001.
Even at a high shear rate, these low concentrations (0.2 and 0.5 µM) of ibrutinib effectively reduced platelet thrombi on plaque homogenate (−69 ± 16% and −93 ± 5%, respectively), whereas platelet aggregation onto collagen was not compromised (−1.2 ± 5% and 1.8 ± 2%, respectively) (Figure 2B).
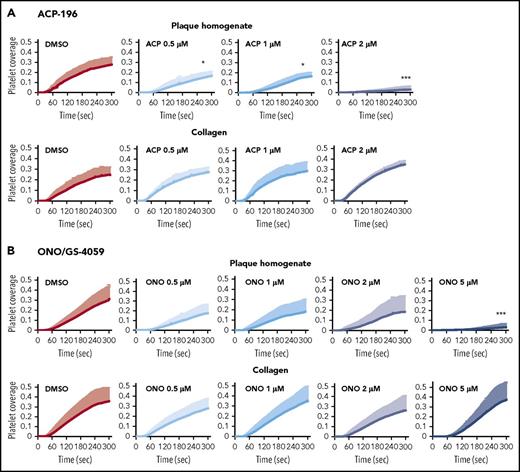
Similarly, acalabrutinib (0.5, 1 and 2 µM) dose-dependently inhibited platelet aggregation on plaque homogenate but not on collagen (Figure 3; supplemental Figure 7). Acalabrutinib (2 µM) suppressed platelet aggregation on plaque homogenate (−90 ± 12%) but not on collagen (25 ± 29%). ONO/GS-4059 (5 µM) blocked platelet aggregation on plaque homogenate (−91 ± 13%), but not on collagen (5 ± 50%), under arterial flow (Figure 3; supplemental Figure 7). In terms of concentrations, ibrutinib was more potent than acalabrutinib and ONO/GS-4059 (supplemental Figure 7).
Acalabrutinib (ACP-196) and ONO/GS-4059 inhibit plaque-induced, but not collagen-induced, platelet thrombus formation under arterial flow. Blood samples were incubated with DiOC6 for platelet labeling and with solvent (0.1% DMSO) or increasing concentrations of ACP-196 (ACP) or ONO/GS-4059 (ONO) (all in DMSO, final concentration of 0.1%) for 15 minutes before the start of blood flow at a shear rate of 600/s. The line graphs show the effects of ACP (A) and ONO (B) on the kinetics of plaque- and collagen-induced platelet deposition. Data are mean + SD (n = 5 for ACP; n = 6 for 0.5 μM ONO; n = 6 for 1 µM ONO; n = 7 for 2 µM ONO; n = 7 for 5 μM ONO). Statistically significant differences compared with DMSO control are indicated 5 minutes after the start of blood flow. *P < .05, *** P < .001. Other statistically significant differences after pairwise comparisons are ACP 0.5 µM vs ACP 2 μM, P < .05; ONO 5 µM vs ONO 2 µM, P < .001; ONO 5 µM vs ONO 1 µM, P < .001; and ONO 5 µM vs ONO 0.5 µM, P < .001.
Acalabrutinib (ACP-196) and ONO/GS-4059 inhibit plaque-induced, but not collagen-induced, platelet thrombus formation under arterial flow. Blood samples were incubated with DiOC6 for platelet labeling and with solvent (0.1% DMSO) or increasing concentrations of ACP-196 (ACP) or ONO/GS-4059 (ONO) (all in DMSO, final concentration of 0.1%) for 15 minutes before the start of blood flow at a shear rate of 600/s. The line graphs show the effects of ACP (A) and ONO (B) on the kinetics of plaque- and collagen-induced platelet deposition. Data are mean + SD (n = 5 for ACP; n = 6 for 0.5 μM ONO; n = 6 for 1 µM ONO; n = 7 for 2 µM ONO; n = 7 for 5 μM ONO). Statistically significant differences compared with DMSO control are indicated 5 minutes after the start of blood flow. *P < .05, *** P < .001. Other statistically significant differences after pairwise comparisons are ACP 0.5 µM vs ACP 2 μM, P < .05; ONO 5 µM vs ONO 2 µM, P < .001; ONO 5 µM vs ONO 1 µM, P < .001; and ONO 5 µM vs ONO 0.5 µM, P < .001.
In comparison with Btk inhibitors, the Src family kinase inhibitor PD173952 showed inferior selectivity for inhibition of plaque- versus collagen-stimulated platelet aggregate formation (supplemental Figure 8). PD173952 (10 µM) inhibited platelet aggregation on plaque homogenate and plaque tissue, as well as on collagen (−50%) (supplemental Figure 8). Lower concentrations of PD173952 (2 and 5 µM) delayed and reduced platelet aggregation on plaque homogenate and plaque tissue, as well as on collagen (supplemental Figure 8).
Btk inhibitors also reduced platelet adenosine triphosphate secretion in blood stimulated by plaque homogenate by ∼90% (supplemental Figure 9).
Btk inhibition does not affect platelet integrin α2β1 function
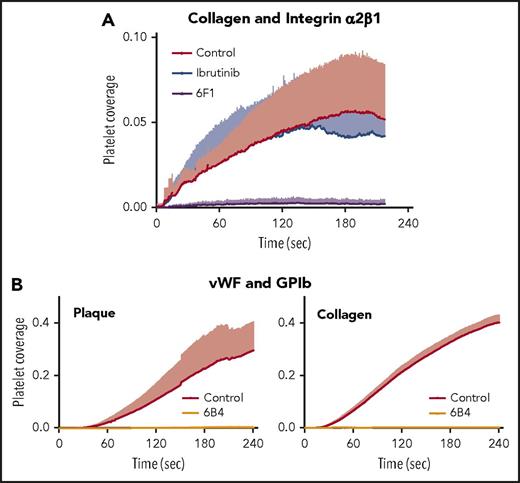
Under arterial flow, the integrin α2β1-blocking antibody 6F1, but not ibrutinib, blocked platelet adhesion to soluble type I collagen (Figure 4A). This confirms that the integrin α2β1 can maintain platelet adhesion to soluble collagen and demonstrates that ibrutinib does not interfere with the integrin α2β1–mediated platelet adhesion to native collagen that is critical for physiologic hemostasis.
Mechanism of plaque-selective inhibition of platelet inhibition under flow by ibrutinib. (A) The anti-α2β1 antibody 6F1, but not ibrutinib, inhibits platelet adhesion onto immobilized soluble collagen under arterial flow. Blood was incubated for 15 minutes at 37°C before the start of flow (shear 600/s) with DiOC6, abciximab (20 µg/mL), and DMSO (0.1%; Control), ibrutinib (1 µM), or 6F1 (20 µg/mL). Data are mean + SD (n = 4). (B) The anti-GPIbα antibody 6B4 inhibits plaque- and collagen-induced platelet aggregate formation under arterial flow. Blood samples were incubated with DiOC6, in the absence or presence of 6B4 (5 µg/mL), before perfusion at a high shear rate (1500/s). Data are mean + SD (n = 4 or 5).
Mechanism of plaque-selective inhibition of platelet inhibition under flow by ibrutinib. (A) The anti-α2β1 antibody 6F1, but not ibrutinib, inhibits platelet adhesion onto immobilized soluble collagen under arterial flow. Blood was incubated for 15 minutes at 37°C before the start of flow (shear 600/s) with DiOC6, abciximab (20 µg/mL), and DMSO (0.1%; Control), ibrutinib (1 µM), or 6F1 (20 µg/mL). Data are mean + SD (n = 4). (B) The anti-GPIbα antibody 6B4 inhibits plaque- and collagen-induced platelet aggregate formation under arterial flow. Blood samples were incubated with DiOC6, in the absence or presence of 6B4 (5 µg/mL), before perfusion at a high shear rate (1500/s). Data are mean + SD (n = 4 or 5).
Plaque- and collagen-induced platelet aggregation at high shear arterial flow requires the binding of VWF to GPIb
Previous studies have shown that Btk is involved in VWF/GPIb signaling under static conditions and in platelet adhesion to immobilized VWF under arterial flow in mice and man.16,32,33 To further dissect the mechanism of plaque-specific inhibition of platelet aggregation by ibrutinib at high shear flow, blood was preincubated with the monoclonal antibody 6B4, which inhibits the interaction of GPIbα with VWF.14 When perfused over plaque homogenate and collagen at high shear, plaque homogenate- and collagen-induced platelet thrombus formation was inhibited equally well (>95%) (Figure 4B). This indicates that VWF binding to GPIb is required for plaque and collagen stimulation of platelets. Our findings also imply that GPIb-induced Btk activation is not required for platelet thrombus formation on native collagen, where other signaling molecules may compensate for inhibition of Btk (Figure 2B).
Plaque- and collagen-induced platelet aggregation under static and arterial flow conditions in patients on ibrutinib therapy
Next, we analyzed platelet function in 5 CLL patients on ibrutinib therapy (420 mg/d) compared with 5 matched patients (4 CLL patients, 1 MCL patient) not taking ibrutinib. Blood platelet counts were similar in patients on ibrutinib (174 × 109 ± 19 × 109/L) and in controls (186 × 109 ± 37 × 109/L). In blood from patients on ibrutinib, static platelet aggregation stimulated by plaque or collagen was inhibited (−89 ± 7% and −84 ± 8%, respectively) (supplemental Figure 10).
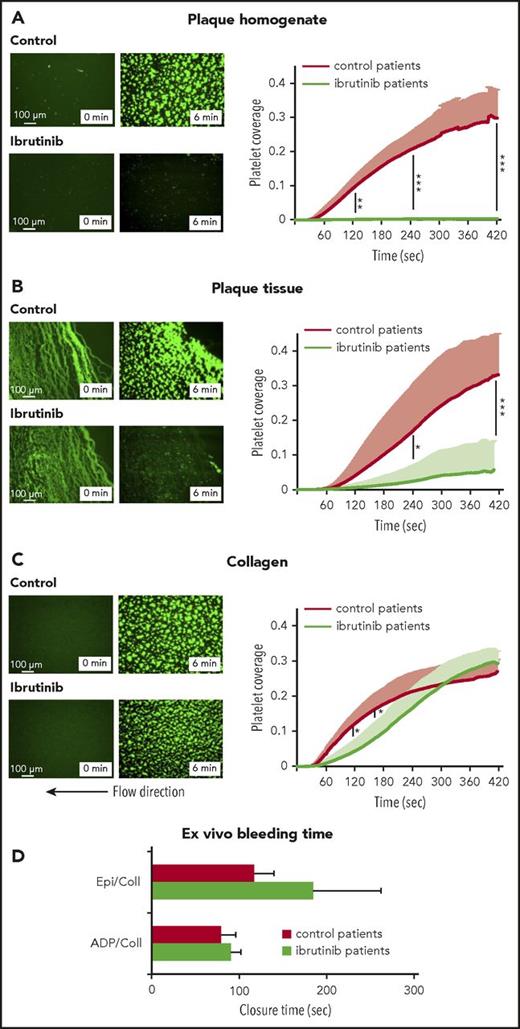
Of note, ibrutinib treatment abolished platelet thrombus formation on plaque homogenate under arterial flow conditions (Figure 5A; supplemental Videos 1 and 2). Also platelet thrombus formation on plaque tissue was largely prevented (−83 ± 24%; Figure 5B; supplemental Videos 3 and 4). In contrast, ibrutinib only minimally slowed initial platelet aggregation from flowing blood onto collagen fibers, which finally reached the same maximal response (108 ± 13%) as in controls (Figure 5C; supplemental Videos 5 and 6).
Inhibition of platelet thrombus formation on atherosclerotic plaque, but not collagen, under arterial flow in patients treated with ibrutinib. Representative micrographs show platelet coverage of plaque homogenate (A), plaque tissue sections (B), and collagen (C) at 0 and 6 minutes after the start of flow (shear rate 600/s) with blood from ibrutinib-treated patients and control patients (left panels). Effects of ibrutinib treatment on the kinetics of plaque- and collagen-induced platelet deposition (right panels). Data are mean + SD (n = 5). (D) Effect of oral ibrutinib treatment on bleeding time in vitro (PFA-100). Blood samples from 5 patients treated with ibrutinib and 5 control patients were transferred to epinephrine/collagen (Epi/Coll) or ADP/collagen (ADP/Coll) cartridges, and the in vitro CT was measured with a PFA-100 platelet function analyzer. Data are mean + SD (n = 5). *P < .05, **P < .01, ***P < .001.
Inhibition of platelet thrombus formation on atherosclerotic plaque, but not collagen, under arterial flow in patients treated with ibrutinib. Representative micrographs show platelet coverage of plaque homogenate (A), plaque tissue sections (B), and collagen (C) at 0 and 6 minutes after the start of flow (shear rate 600/s) with blood from ibrutinib-treated patients and control patients (left panels). Effects of ibrutinib treatment on the kinetics of plaque- and collagen-induced platelet deposition (right panels). Data are mean + SD (n = 5). (D) Effect of oral ibrutinib treatment on bleeding time in vitro (PFA-100). Blood samples from 5 patients treated with ibrutinib and 5 control patients were transferred to epinephrine/collagen (Epi/Coll) or ADP/collagen (ADP/Coll) cartridges, and the in vitro CT was measured with a PFA-100 platelet function analyzer. Data are mean + SD (n = 5). *P < .05, **P < .01, ***P < .001.
Effect of ibrutinib ex vivo on in vitro CT
To test whether ibrutinib might increase bleeding time, we performed measurements using the platelet function analyzer PFA-100, which simulates primary hemostasis in vitro, reflecting potential hemorrhagic risk.31,34 Two of the 5 patients on ibrutinib had a closure time (CT) above the normal range when measured with the collagen/epinephrine test cartridge, but the mean CT was not significantly increased (Figure 5D). With the collagen/ADP test cartridge, CT was in the normal range in all patients (Figure 5D).
Oral intake of low doses of ibrutinib inhibits plaque-induced platelet aggregation under static conditions and arterial flow
Because Btk inhibitors irreversibly inactivate the enzyme, and anucleate platelets lack de novo synthesis of most proteins, we explored whether in vivo low and intermittent dosing might be sufficient for platelet inhibition. Two healthy male physicians took a loading dose of ibrutinib (3 × 140 mg) on day 1, followed by 140 mg/d for 1 week (donor A) or 140 mg on alternate days for 1 week (donor B). At 3 hours after intake of 420 mg of ibrutinib, static platelet aggregation was inhibited by 94% on 2 plaque homogenate pools and by 91% on collagen in donor A (data not shown). In donor B, platelet aggregation was reduced by 73% on plaque stimulation and by 58% on collagen stimulation (Figure 6A). Platelet aggregation induced by TRAP, ADP, or AA was preserved in donors A and B (data not shown, Figure 6A).
Effect of oral ibrutinib intake (loading dose and low maintenance dose) on platelet aggregation in static and flow assays in 2 volunteers. (A) Rapid suppression of plaque-induced platelet aggregation by oral intake of 420 mg of ibrutinib. Blood was collected before (control) and 3 hours after the intake of 3 × 140 mg of ibrutinib (donor B). (B) Platelet inhibition is maintained by low-dose ibrutinib for 1 week. Blood was collected after the intake of 140 mg/d of ibrutinib (donor A) or 140 mg of ibrutinib on alternate days (donor B) for 1 week. Static platelet aggregation induced with 2 pools of plaque homogenate (A and B; 833 μg/mL), collagen (0.2 μg/mL), TRAP (5 μM), ADP (5 μM), or AA (0.6 mM) (left panels). Kinetics of plaque- and collagen- induced platelet thrombus formation under arterial flow (600/s) in donor A and donor B (right panels). Data are mean + SD of triplicates.
Effect of oral ibrutinib intake (loading dose and low maintenance dose) on platelet aggregation in static and flow assays in 2 volunteers. (A) Rapid suppression of plaque-induced platelet aggregation by oral intake of 420 mg of ibrutinib. Blood was collected before (control) and 3 hours after the intake of 3 × 140 mg of ibrutinib (donor B). (B) Platelet inhibition is maintained by low-dose ibrutinib for 1 week. Blood was collected after the intake of 140 mg/d of ibrutinib (donor A) or 140 mg of ibrutinib on alternate days (donor B) for 1 week. Static platelet aggregation induced with 2 pools of plaque homogenate (A and B; 833 μg/mL), collagen (0.2 μg/mL), TRAP (5 μM), ADP (5 μM), or AA (0.6 mM) (left panels). Kinetics of plaque- and collagen- induced platelet thrombus formation under arterial flow (600/s) in donor A and donor B (right panels). Data are mean + SD of triplicates.
After 1 week of low-dose ibrutinib intake, platelet aggregation stimulated by the 2 plaque homogenate pools was inhibited by 94% and 91% in donor A and by 93% and 91% in donor B (Figure 6B). Also, static platelet aggregation on collagen stimulation was inhibited (91% in donor A, 82% in donor B), but it was barely affected after ADP or AA stimulation (Figure 6B). TRAP-induced platelet aggregation was reduced more in donor A than in donor B. Plaque-induced platelet aggregation was measured on several days during the intake of ibrutinib (140 mg/d) by donor A and was always found to be inhibited >90%, independent of whether the blood was sampled 23 or 3 hours after taking the drug (data not shown).
Three hours after intake of 420 mg of ibrutinib, platelet thrombus formation under arterial flow conditions was suppressed on plaque, but not on collagen, in both donors (data not shown, Figure 6A). After 1 week, the low dose and the very low dose of ibrutinib suppressed plaque-induced, but not collagen-induced, platelet thrombus formation (Figure 6B, right panels).
Ibrutinib intake (loading dose or 1-week low-dose intake) did not affect red cell, platelet, or white blood cell (lymphocyte, neutrophil, monocyte) counts. B-lymphocytes were increased, rather than decreased, in both donors (supplemental Table 1). Liver enzymes were not altered by ibrutinib intake (data not shown).
Low-dose ASA (100 mg daily) for 1 week reduced platelet aggregation stimulated by the 2 plaque homogenate pools, A and B, less effectively than did low-dose ibrutinib: by 49% in donor A and by 24% in donor C (supplemental Figure 11, left panels). Platelet aggregation upon TRAP stimulation was reduced by 63% and 52% in donors A and C by ASA. ASA reduced, as expected, AA-stimulated platelet aggregation by 78% in donor A and by 74% in donor C, similar to previous findings.35 After 1 week of low-dose ASA, plaque-induced platelet thrombus formation at 4.5 minutes was reduced in donor A by 64% and in donor C by 53% (supplemental Figure 11, right panels), but it was not suppressed as much as after low-dose ibrutinib intake (Figure 6B, right panels).
Discussion
Antithrombotic compounds developed for cardiovascular use often have not fulfilled expectations that thrombosis, but not hemostasis, will be inhibited. We demonstrate here that the Btk inhibitors ibrutinib, acalabrutinib, and ONO/GS-4059 selectively block activation of platelets exposed to atherosclerotic plaque material under arterial flow, but they spare integrin α2β1– and VWF/GPIb-mediated platelet adhesion and aggregation on collagen, which is important for physiologic hemostasis. Static platelet aggregation induced by ADP and AA was, at most, marginally reduced by Btk inhibitors. Importantly, this plaque-selective platelet inhibition could be demonstrated in patients taking ibrutinib and was seen in human volunteers taking doses of ibrutinib that are considerably lower than the currently tolerated therapeutic doses. Thus, oral covalent Btk inhibitors applied at low dosage might offer a novel antiplatelet therapy to selectively target thrombus growth on injured plaques.
Ibrutinib and the new more-selective irreversible Btk inhibitors without off-target kinase effects (eg, acalabrutinib and ONO/GS-4059) are successfully used for the treatment of CLL and MCL. For ibrutinib, long-term safety data from a large number of patients are available.36 The most common adverse events include diarrhea, fatigue, and nausea. Low-grade bleeding events (spontaneous bruising, epistaxis, or petechiae) have been observed in 50% of patients, but major bleeding is rarely observed in patients treated with high-dose ibrutinib.33,36,37 The clinical trials with acalabrutinib and ONO/GS-4059 reported only infrequent low-grade bleeding (petechiae),22,23 and they are apparently better tolerated than ibrutinib.22,38
We demonstrate that ibrutinib, acalabrutinib, and ONO/GS-4059 in vitro and ibrutinib ex vivo inhibit GPVI-mediated plaque- and collagen-triggered platelet aggregation in blood under static conditions, whereas ADP- or AA-induced static platelet aggregation was, at most, marginally reduced, even at high drug concentrations. In 2 previous studies using platelet-rich plasma, ibrutinib also inhibited platelet aggregation induced by collagen but not ADP.32,39
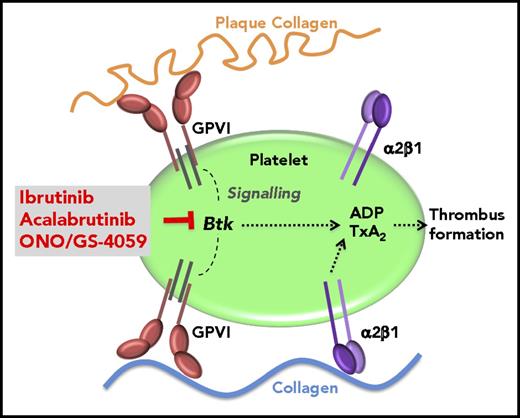
Moreover, if tested in flowing blood perfused over plaque homogenate or sections, ibrutinib caused a profound inhibition of plaque-stimulated aggregation, whereas platelet arrest on immobilized native collagen at shear rates present in intact (600/s; Figures 2A and 5) or mildly stenotic (1500/s; Figure 2B) atherosclerotic coronary arteries was not affected. A similar differential platelet inhibition was demonstrated with acalabrutinib and ONO/GS-4059 (Figure 3; supplemental Figure 7). What might underlie this plaque and flow selectivity of the Btk inhibitors? We found that ibrutinib did not inhibit the integrin α2β1–dependent platelet adhesion to immobilized soluble collagen under arterial flow. During arterial flow over native collagen fibers, the platelet integrin α2β1 is critical in initial platelet arrest to collagen, and it plays a role complementary to GPVI in ensuing platelet aggregation on collagen (Figure 7).8,9,15,25 This is essential for physiologic hemostasis after disruption of vessel wall continuity, as in trauma exposing native collagen. In contrast, injured plaque exposes structurally modified collagen, and platelet accretion from flowing blood depends on platelet GPVI but not integrin α2β1 (Figure 7).2,5
Model of the plaque-selective inhibition of platelet thrombus formation by Btk inhibitors. TxA2, thromboxane A2.
Model of the plaque-selective inhibition of platelet thrombus formation by Btk inhibitors. TxA2, thromboxane A2.
Plaque homogenates and plaque tissue sections in which the plaque architecture is preserved yielded similar results. The subtle roughness of the plaque sections might indeed closely model the rough thrombogenic surface generated by rupture of thin-capped fibroatheroma, exposing the necrotic core with distorted collagenous structures to circulating blood.5,40
Our experiments were performed in the absence of coagulation. In the presence of coagulation, the major thrombogenic plaque components, collagen and tissue factor (TF), induce platelet activation and coagulation, respectively, in 2 consecutive steps: GPVI-mediated platelet adhesion and aggregation to plaque collagen precedes TF-stimulated thrombin and fibrin formation.4 Targeting the first step was crucial to inhibit thrombus formation.4 Btk inhibitors are the first oral drugs that specifically inhibit this initial trigger of atherothrombosis.
Btk also plays a role in VWF/GPIb signaling and platelet adhesion under arterial flow to immobilized VWF in mice and man.16,32,33 At a shear rate of 1500/s, ibrutinib abolished platelet deposition on plaque, but it had only a marginal insignificant effect on native collagen-induced platelet deposition (Figure 3). Therefore, GPIb-induced Btk activation is obviously not essential for platelet thrombus formation on native collagen, but it may contribute to platelet recruitment onto atherosclerotic plaque at high shear rate.
It has also been reported that Btk downstream of integrin aIIbβ3 is activated in thrombin-aggregated washed platelets and that ibrutinib inhibits platelet integrin αIIbβ3 outside-in signaling of immobilized fibrinogen.41,42 This might explain the inhibition of spontaneous platelet aggregation and the, at most, 30% reduction in PAR-1 (TRAP, 5 µM) by Btk inhibitors in static assays in vitro (Figure 1; supplemental Figure 2), as well as the variable reduction in TRAP (5 µM)–induced platelet aggregation after low-dose ibrutinib intake (Figure 6). In comparison, approved doses of the PAR-1 antagonist vorapaxar (2.5 mg/d) inhibit platelet aggregation in blood by 93%, even when stimulated with high TRAP concentrations (15 µM).43 PAR-4–stimulated platelet aggregation was barely affected by Btk inhibitors (supplemental Figure 3). Therefore, inhibition of thrombin-induced platelet aggregation in blood by Btk inhibitors is likely to be small. In addition, these mechanisms are apparently not relevant after platelet stimulation with ADP or AA (Figure 1).
Comparing the platelet-inhibitory effects of ibrutinib, acalabrutinib, and ONO/GS-4059, we found no principal differences; however, for ibrutinib, off-target effects on Tec (belonging to the Tec family–like Btk) and the Src kinase Yes, which are expressed in platelets, have also been reported.22 Acalabrutinib only inhibits Btk, whereas ONO/GS-4059 also inhibits Tec.22,23 Collagen-induced aggregation of platelets from mice deficient in Btk and Tec was found to be more impaired than aggregation of platelets from single Btk–deficient mice, suggesting an additional effect by inhibiting Tec and Btk.44 We observed that the potency of Btk inhibitors decreased in the order ibrutinib > acalabrutinib > ONO/GS-4059. It should be emphasized that the concentrations of acalabrutinib and ONO/GS-4059 that are effective in vitro are reached in blood after oral therapeutic doses.22,23,45
Our study suggests that targeting Btk downstream of GPIb and GPVI holds promise to effectively suppress occluding acute atherothrombosis locally, with little impairment of physiologic hemostasis. The mean CTs in the PFA-100 device simulating primary hemostasis were not increased significantly in patients on ibrutinib therapy; only 2 patients showed a prolonged CT with the collagen/epinephrine cartridge. Such a delayed CT is usually observed on ASA.34 A recent study of new ibrutinib analogs administered orally to nonhuman primates also did not show an increased template skin bleeding time.46 Importantly, X-linked agammaglobulinemia patients with genetic Btk deficiency do not have a bleeding phenotype.47,48
Our small pilot study shows that much lower doses of Btk inhibitors than used for CLL may suffice for antiplatelet therapy. A preferential Btk inhibition in platelets is, like with ASA, achievable in vivo by exploiting the lack of de novo enzyme synthesis in platelets and the covalent binding of Btk inhibitors to Btk. A pulsed regimen of low-dose Btk inhibitors analogous to ASA49 permanently inactivates Btk in platelets passing through the portal circulation during the absorption phase and, thus, suppresses the key mechanism for plaque-initiated thrombus formation in platelets for their life span; however, it allows other cells to rapidly resynthesize Btk. Thus, low dosing of ibrutinib and higher Btk selectivity of newer inhibitors might further reduce potential side effects.
We demonstrate a selective and very potent suppression of platelet thrombus formation on human atherosclerotic plaque by several Btk inhibitors, whereas hemostatic platelet function was spared. These results, together with the growing clinical experience of the long-term safety profiles of selective Btk inhibitors, should encourage further studies of their potential as the first available oral antithrombotic compounds targeting GPIb and GPVI, such as in elective coronary interventions, inevitably exposing plaque material, for which 3 systemic antithrombotic compounds (heparin, ASA, and P2Y12 antagonists) are currently required. We suggest that, in these situations, the culprit lesion–focused platelet inhibition of a low-dose Btk inhibitor, in combination with a classic antiplatelet drug (ASA and/or a P2Y12 antagonist) and heparin (inhibiting plaque TF-induced thrombin formation), will improve antiatherothrombotic efficacy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. von Oheimb for expert technical assistance and R. Megens for video editing.
This work was supported by grants from the August-Lenz Foundation and the Deutsche Forschungsgemeinschaft (SFB1123/B08).
The results are part of the doctoral thesis of K.B. at the University of Munich.
Authorship
Contribution: K.B. and J.J. contributed to research design and acquired and analyzed the data; K.B. and W.S. drafted the manuscript; T.S. recruited patients and revised the manuscript; R.B. performed surgical endarterectomy and was critically involved in the handling and processing of atherosclerotic plaque material; H.D. provided key reagents and critically revised the manuscript; C.W. and W.S. handled funding and supervised the research; R.L. performed statistical analyses and contributed to the writing of the manuscript; and W.S. conceived the project and designed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfgang Siess, Institut für Prophylaxe und Epidemiologie der Kreislaufkrankheiten, Klinikum Innenstadt, Ludwig-Maximilians-Universität München, Pettenkoferstr 9, D-80336 Munich, Germany. e-mail: wsiess@med.uni-muenchen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal