TO THE EDITOR:
Light-chain amyloidosis (AL) is a rare systemic protein deposition disorder that belongs to the group of plasma cell dyscrasias. Approximately 10% to 22% of cases are associated with symptomatic multiple myeloma or Waldenström macroglobulinemia (WM). The disease is characterized by extracellular deposition of insoluble fibrils composed of misfolded molecules of immunoglobulin light chains produced by a clonal B-lymphocyte population. Immunoglobulin M (IgM) AL represents only about 5% of patients of the entire AL spectrum. These patients exhibit lower serum levels of amyloidogenic light chains and cardiac biomarkers than non-IgM AL patients.1-3 Considering the indolent character of WM, it is necessary to point out that in cases of AL due to WM, AL is the dominant cause of death. Although protocols for multiple myeloma therapy have been successfully implemented for the treatment of non-IgM AL, experience with protocols used for WM is scarce in the treatment of IgM AL. Chlorambucil and prednisone combination was associated with 27% to 38% of therapeutic responses, whereas purine analog therapy led to 73%.1,3,4 Autologous transplantation yielded a response in 89% of 12 evaluated patients.5 Regarding application of biologically active products, Palladini et al report a 78% therapeutic response for the combination of rituximab, bortezomib, and dexamethasone, including patients with relapsed/refractory disease.6 For the monitoring of a therapeutic response in AL, measuring levels of free light chains (FLCs) is dominantly used. Hematologic response criteria are defined as negative serum and urine immunofixation with normal FLC ratio in complete response (CR), difference of amyloidogenic and uninvolved FLCs in the serum (dFLC) <40 mg/L in very-good partial response (VGPR), and dFLC decrease >50% in partial response (PR).7 The therapeutic goal for patients with AL is a reduction in levels of amyloidogenic FLC to at least VGPR, which is associated with significant improvement of patient survival and organ response (OR) shown for AL overall and IgM AL.1,7
Ibrutinib is currently approved and registered for treatment of WM (and also chronic lymphocytic leukemia and mantle cell lymphoma). Treon et al report 90.5% therapeutic response in the case of relapsed/refractory WM, with 73% major therapeutic response (16% VGPR and 57% PR), but interestingly no patient achieved CR.8,9 M-protein response criteria in WM are defined as disappearance of IgM spike by immunofixation, negative bone marrow examination, and resolution of extramedullary disease in CR, >90% reduction of M-protein and resolution of extramedullary disease in VGPR, and >50% M-protein reduction in PR from baseline with reduction in extramedullary disease.10 Importantly, FLCs are not part of the usual diagnostic setup, and therefore dFLC response data are lacking in WM. The most frequent and largest therapeutic responses were seen in patients with MYD88 L265P mutation without mutation of CXCR4. Principal adverse effects of the treatment include blood count changes (thrombopenia and neutropenia), hemorrhagic symptoms, and atrial fibrillation. Dimopoulos et al reported a 90% total therapeutic response rate (71% major response) in rituximab-refractory patients. The most frequent side effects were blood count changes, diarrhea, and arterial hypertension.11 In a preclinical study, Cao et al reported that the WM cells carrying the WHIM-like CXCR4S338X mutation are more resistant to ibrutinib.12
In our series, we retrospectively evaluated the effect of ibrutinib therapy in 8 patients with AL associated with WM or marginal zone lymphoma, treated or evaluated at the amyloidosis centers at Heidelberg (Germany) and Olomouc (Czech Republic) from 2014 to 2016. Patients signed an informed consent with data processing. All patients were men, aged 52 to 74 years at the time when AL was diagnosed, whose WM diagnosis preceded the AL diagnosis by 0 to 12 years. At the time of AL diagnosis, IgM λ represented the dominant M-protein type (5 of 8), median M-protein level was 18.8 g/L (range, 4.9-33.2 g/L), and median dFLC level was 312.7 mg/L (range, 90-960 mg/L); 3 patients manifested palpable lymph nodes and spleen (Table 1). Detection of MYD88 mutation was carried out in 7 patients, with 2 negative results. CXCR4WT status was detected in all 4 analyzed patients. According to the consensus criteria, amyloid cardiomyopathy was identified in 5 of 8 and nephropathy in 3 of 8 patients. One patient had peripheral neuropathy, 1 had lung involvement, and soft-tissue infiltration was identified in 4 patients. Four patients underwent 1 line of therapy, 3 patients underwent 2 lines, and 1 patient even underwent 3 lines of therapy before treatment with ibrutinib. The combination of rituximab and bendamustine was used as the initial regimen in 6 of 8 of the patients. Hematologic therapeutic responses were achieved in 5 of 8 of the patients (4 PR, 1 VGPR) before ibrutinib; 3 patients were primarily refractory.
IgM-related amyloidosis patients treated with ibrutinib
| Pt. . | Lines before AL . | Lymphoma type . | MYD88/ CXCR4 . | Organ involvement (Mayo stage) . | Previous lines . | Best response . | Time from AL diagnosis to ibrutinib treatment, mo . | Ibrutinib response treatment duration, mo . | Adverse events (CTCAE grade), onset since ibrutinib start . | Hematologic toxicity (grade) . | Next line . | Death (cause), survival or follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | None | WM | MUT/WT | Heart (3), lung | R/B, BRD | PR | 7 | SD/NR, 3 | TIA (3), 1 mo | None | FCR | Yes (stroke), 5 |
| 2 | 1 | WM | MUT/ND | Heart (1) | R/B, CFA | VGPR | 24 | VGPR with OR, 16 | PNP (1), 2 mo | Thrombopenia, anemia (1) | CFA | No, 25 |
| 3 | 1 | WM/MZL | MUT/ND | Kidney, soft tissue | R/CVD, Len/dex | PR | 18 | PD, 2 | Edema (kidney 3), 1 mo | None | ASCT | Yes (sepsis), 11 |
| 4 | 1 | WM | MUT/WT | Heart (ND), soft tissue | R/B | SD/NR | 19 | SD/NR, 6 (continue) | Edema (cardiac 2), 2 mo | Anemia (1) | None | No, 6 |
| 5 | 2 | WM | WT/WT | Heart (3), kidney | CVD | SD/NR | 16 | PR, 9 | PNP (1), diarrhea (1), 1 mo | Thrombopenia (2) | None | Yes (cardiac death), 9 |
| 6 | 1 | WM/MZL | WT/WT | Kidney, liver, PNP | R/B | PR | 22 | SD/NR, 3 | Edema (kidney 1), skin bleeding (1), 2 wk | Anemia (1) | None | Yes (AL progression),9 |
| 7 | None | WM | MUT/ND | Heart (3), soft tissue | R/B | SD/NR | 4 | SD/NR, 2 | Edema (cardiac 3) Atrial fibrillation (3), 2 wk | None | M-dex | Yes (AL progression), 2 |
| 8 | None | WM | ND/ND | Soft tissue | R/B, Len/dex, Vel/dex | PR | 57 | SD/NR, 5 | Atrial fibrillation (2), Pleural effusion (3), 3 mo | None | None | No, 9 |
| Pt. . | Lines before AL . | Lymphoma type . | MYD88/ CXCR4 . | Organ involvement (Mayo stage) . | Previous lines . | Best response . | Time from AL diagnosis to ibrutinib treatment, mo . | Ibrutinib response treatment duration, mo . | Adverse events (CTCAE grade), onset since ibrutinib start . | Hematologic toxicity (grade) . | Next line . | Death (cause), survival or follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | None | WM | MUT/WT | Heart (3), lung | R/B, BRD | PR | 7 | SD/NR, 3 | TIA (3), 1 mo | None | FCR | Yes (stroke), 5 |
| 2 | 1 | WM | MUT/ND | Heart (1) | R/B, CFA | VGPR | 24 | VGPR with OR, 16 | PNP (1), 2 mo | Thrombopenia, anemia (1) | CFA | No, 25 |
| 3 | 1 | WM/MZL | MUT/ND | Kidney, soft tissue | R/CVD, Len/dex | PR | 18 | PD, 2 | Edema (kidney 3), 1 mo | None | ASCT | Yes (sepsis), 11 |
| 4 | 1 | WM | MUT/WT | Heart (ND), soft tissue | R/B | SD/NR | 19 | SD/NR, 6 (continue) | Edema (cardiac 2), 2 mo | Anemia (1) | None | No, 6 |
| 5 | 2 | WM | WT/WT | Heart (3), kidney | CVD | SD/NR | 16 | PR, 9 | PNP (1), diarrhea (1), 1 mo | Thrombopenia (2) | None | Yes (cardiac death), 9 |
| 6 | 1 | WM/MZL | WT/WT | Kidney, liver, PNP | R/B | PR | 22 | SD/NR, 3 | Edema (kidney 1), skin bleeding (1), 2 wk | Anemia (1) | None | Yes (AL progression),9 |
| 7 | None | WM | MUT/ND | Heart (3), soft tissue | R/B | SD/NR | 4 | SD/NR, 2 | Edema (cardiac 3) Atrial fibrillation (3), 2 wk | None | M-dex | Yes (AL progression), 2 |
| 8 | None | WM | ND/ND | Soft tissue | R/B, Len/dex, Vel/dex | PR | 57 | SD/NR, 5 | Atrial fibrillation (2), Pleural effusion (3), 3 mo | None | None | No, 9 |
The Mayo 2004 staging system was used.
ASCT, autologous stem cell transplantation; BRD, bortezomib, rituximab, dexamethasone; CFA, cyclophosphamide; CTCAE, Common Terminology Criteria for Adverse Events; CVD, cyclophosphamide, bortezomib, dexamethasone; FCR, fludarabine, cyclophosphamide, rituximab; Len/dex, lenalidomide, dexamethasone; M-dex, melphalan, dexamethasone; MUT, mutated; MZL, marginal zone lymphoma; ND, not done; PNP, polyneuropathy; Pt., patient; R/B, rituximab, bendamustine; TIA, transient ischemic attack; Vel/dex, bortezomib, dexamethasone; WT, wild type.
Response criteria: OR, organ response; PD, progressive disease; SD/NR, stable disease/no response.
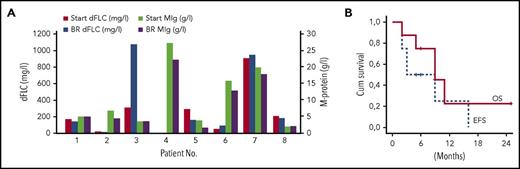
Ibrutinib was initiated with a standard recommended dose of 420 mg per day after nonsatisfying response or progression after previous treatment. The medians of initial M-protein and dFLC level were 5.8 g/L (range, 1.8-27.5 g/L) and 203 mg/L (range, 17-902 mg/L), respectively. Median therapy duration was 4 months (2-16 months), and ibrutinib led to a hematologic dFLC response in only 2 patients.7 The first patient who had already achieved VGPR (CXCR4 not tested) before ibrutinib exhibited a 40% reduction of the cardiac biomarker N-terminal prohormone of brain natriuretic peptide compared with baseline values (OR) and sustained these responses for 16 months. The other patient (CXCR4WT) who achieved a hematologic PR with ibrutinib also exhibited a decrease of initial lymphadenopathy, but no amyloidosis OR. The treatment did not affect the levels of amyloidogenic light chains or complete M-protein molecules in 5 patients; 1 patient even had a serological progression under treatment (Figure 1A). Median levels of M-protein and dFLC at best response were 4.7 g/L (range, 1.5-22.3 g/L) and 158 mg/L (range, 12-947 mg/L), respectively. Median overall survival of the whole cohort was 9 months with a median follow-up of 6 months in surviving patients (Figure 1B) whereas the median event-free survival was only 3 months. The most frequent adverse events were peripheral edema especially in patients with cardiac and kidney involvement, and polyneuropathy. Two patients developed atrial fibrillation and 1 patient with preexisting atrial fibrillation experienced a transient ischemic attack. Mild hematologic toxicity (anemia, thrombocytopenia) was seen in 4 of 8 patients, only in 1 with skin bleeding. We did not observe significant change in renal and hepatic functions during ibrutinib therapy. Levels of the N-terminal prohormone of brain natriuretic peptide were evaluated in 4 of 8 patients with no increase. However, in advanced AL patients, it is often difficult to distinguish between amyloid organ–related complications and side effects of a given drug, especially if the drug is not leading to a fast hematologic response.
Hematologic response and survival in IgM-related amyloidosis. (A) Changes in monoclonal immunoglobulin levels (MIg) and serum free chain levels (dFLC) during ibrutinib therapy in individual patients. (B) Overall survival (OS) and event-free survival (EFS) in patients with IgM-associated AL treated with ibrutinib. Event defined as death, relapse/progression, or next therapy. BR, best response; Cum, cumulative.
Hematologic response and survival in IgM-related amyloidosis. (A) Changes in monoclonal immunoglobulin levels (MIg) and serum free chain levels (dFLC) during ibrutinib therapy in individual patients. (B) Overall survival (OS) and event-free survival (EFS) in patients with IgM-associated AL treated with ibrutinib. Event defined as death, relapse/progression, or next therapy. BR, best response; Cum, cumulative.
Compared with non-IgM amyloidoses, IgM AL is a specific condition associated with a B-lymphoproliferative disease, most often WM. Treatment of refractory/relapsed patients tends to be difficult due to few responses. Although ibrutinib treatment is associated with a high percentage of therapeutic responses in WM, we observed only a few hematologic and organ responses in IgM AL and a considerable amount of adverse effects translating into poor survival in this small subgroup of pretreated systemic AL. The effect of MYD88 and CXCR4 mutations on IgM AL treatment will have to be further investigated, and combination therapies including ibrutinib should also be considered.
Authorship
Contribution: T.P. and S.O.S. collected the data and wrote the manuscript; U.H. and C.K. collected the data; B.M. performed molecular diagnostics; P.F. performed histology; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan O. Schönland, Medical Department V, Amyloidosis Center, University Hospital Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: stefan.schoenland@med.uni-heidelberg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal