TO THE EDITOR:
Systemic amyloid light-chain amyloidosis (AL) is characterized by deposition of misfolded immunoglobulin light chains within organs. AL with an immunoglobulin M (IgM) monoclonal protein (IgM-AL) accounts for 5% to 7% of AL and exhibits more prevalent lymph node, neuropathic, and lung involvement, less prevalent cardiac involvement, and lower amyloidogenic light chains.1 A reduction in intact monoclonal protein and free light chains is prognostic.1 A very good partial response (VGPR) is associated with better survival and organ responses but is challenging to achieve because of an unclear underlying diagnosis (usually lymphoplasmacytic lymphoma) and lack of treatment uniformity. In a large retrospective study, we reported the use of 22 front-line regimens.1 Rituximab-based regimens are often used alongside bortezomib, alkylators, or purine analogs; outcomes remain poor, with few complete responses.1-3 Excellent hematological responses were reported in 12 IgM-AL patients treated with autologous stem cell transplantation,4 but given the older age of IgM-AL patients, this is often not an option. Treatment of relapsed/refractory patients is particularly challenging, and recent experience with ibrutinib was disappointing.5
Bendamustine, which has features of an alkylator and purine analog, is used alongside rituximab in newly diagnosed and relapsed/refractory non-Hodgkin lymphoma6 and in relapsed/refractory Waldenstrom macroglobulinemia, with an overall hematological response rate (ORR) of 83.3%.7 Bendamustine in relapsed/refractory AL (with a plasma cell dyscrasia) resulted in disappointing responses.8 There have been no studies focusing on the use of rituximab-bendamustine (BR) in IgM-AL. We report efficacy of BR in untreated and relapsed/refractory IgM-AL.
Twenty-seven patients treated with BR between 2011 and 2017 were identified from the UK National Amyloidosis Centre database; 22 received BR first-line therapy, 5 received BR second-line therapy. Twenty-five patients had a serum IgM M-protein. Two patients had lymphoplasmacytic lymphoma on bone marrow examination but had a serum IgG M-protein (instead of IgM).
AL was confirmed on biopsy immunohistochemistry or proteomic analysis. All patients underwent serial biochemical tests for organ function, cardiac biomarkers, serum free light chains, serum and urine protein electrophoresis and immunofixation, and echocardiography with or without cardiac magnetic resonance imaging (unless contraindicated). Organ involvement was defined by international amyloidosis consensus criteria.9 Hematological responses were assessed according to AL amyloidosis response criteria.10 In patients with nonevaluable serum free light chains (difference between involved and uninvolved light chains <50 mg/L), IgM M-protein was used for assessment.
Rituximab (375 mg/m2) was administered IV on day 1 as per local protocols. Bendamustine (90 mg/m2) was administered IV over 30 minutes on days 1 and 2. Four patients received additional corticosteroid therapy (3 received once-weekly dexamethasone [20 mg], and 1 received once-weekly dexamethasone [10 mg]). BR was repeated every 28 days.
Outcome variables were hematological and organ response, overall survival (OS), progression-free survival (PFS; progression defined as progression to next treatment or death), and time to next treatment (TNT).
Table 1 shows baseline characteristics. Median age was 70 years (56-86 years). Cardiac, renal, liver, peripheral nerve, autonomic nerve, and soft tissue involvement was found in 17 (63%), 17 (63%), 6 (22%), 6 (22%), 4 (15%), and 2 patients (7%), respectively. Lymphadenopathy was radiologically identified in 13 patients (48%); 7 patients (26%) had biopsy-proven lymph node amyloid deposition. The Mayo 2004 cardiac stage was I in 30% of patients, II in 48%, and III in 22%. Median M-protein was 11.5 g/L (1-30 g/L); 19 (70.4%) had evaluable free light chains. Of the remaining patients, all had an IgM M-protein (>5 g/L) enabling response assessment.10
Baseline characteristics (N = 27)
| . | Median (range) or n (%) . |
|---|---|
| Median age (y) | 70 (56-86) |
| Male:female | 16 (59%):11 (41%) |
| NYHA class | |
| 1 | 12 (44%) |
| 2 | 14 (52%) |
| 3 | 1 (4%) |
| 4 | 0 |
| ECOG | |
| 0 | 7 (26%) |
| 1 | 15 (56%) |
| 2 | 5 (18%) |
| 3 | 0 |
| 4 | 0 |
| Cardiac involvement | 17 (63%) |
| Median NT-pro-BNP (ng/L) | 978 (42-5708) |
| Median cardiac troponin T (ng/L) | 31.5 (3-122) |
| Mayo stage | |
| I | 8 (30%) |
| II | 13 (48%) |
| IIIA (NT-pro-BNP ≤8500 ng/L) | 6 (22%) |
| IIIB (NT-pro-BNP >8500 ng/L) | 0 |
| Median systolic blood pressure (mmHg) | 119 (100-164) |
| Median left ventricular wall thickness (mm) | 11 (8.5-18) |
| Median LV ejection fraction (%) | 60 (36-72) |
| Renal involvement | 17 (63%) |
| Median serum creatinine (μmol/L) | 98 (46-493) |
| Median GFR (mL/min) | 68 (10-100) |
| Median proteinuria (g/24 h) | 0.8 (0.1-34.6) |
| Liver involvement | 6 (22%) |
| Median serum bilirubin (μmol/L) | 6 (2-50) |
| Median ALP (U/L) | 105 (25-1879) |
| Soft tissue involvement | 2 (7%) |
| Peripheral nerve involvement | 6 (22%) |
| Autonomic nerve involvement | 4 (15%) |
| Lymph node involvement | 13 (48%) |
| GI involvement | 0 |
| Median number of involved organs | 1 (1-4) |
| Involved light chains | |
| κ | 7 (26%) |
| Λ | 12 (44%) |
| No monoclonal light-chain excess | 8 (30%) |
| Median dFLC (mg/L) | 59.8 (2.2-856.4) |
| IgM κ M-protein, IgM λ M-protein | 11 (44%), 14 (56%) |
| Median serum monoclonal protein (g/L) | 11.5 (1-30) |
| . | Median (range) or n (%) . |
|---|---|
| Median age (y) | 70 (56-86) |
| Male:female | 16 (59%):11 (41%) |
| NYHA class | |
| 1 | 12 (44%) |
| 2 | 14 (52%) |
| 3 | 1 (4%) |
| 4 | 0 |
| ECOG | |
| 0 | 7 (26%) |
| 1 | 15 (56%) |
| 2 | 5 (18%) |
| 3 | 0 |
| 4 | 0 |
| Cardiac involvement | 17 (63%) |
| Median NT-pro-BNP (ng/L) | 978 (42-5708) |
| Median cardiac troponin T (ng/L) | 31.5 (3-122) |
| Mayo stage | |
| I | 8 (30%) |
| II | 13 (48%) |
| IIIA (NT-pro-BNP ≤8500 ng/L) | 6 (22%) |
| IIIB (NT-pro-BNP >8500 ng/L) | 0 |
| Median systolic blood pressure (mmHg) | 119 (100-164) |
| Median left ventricular wall thickness (mm) | 11 (8.5-18) |
| Median LV ejection fraction (%) | 60 (36-72) |
| Renal involvement | 17 (63%) |
| Median serum creatinine (μmol/L) | 98 (46-493) |
| Median GFR (mL/min) | 68 (10-100) |
| Median proteinuria (g/24 h) | 0.8 (0.1-34.6) |
| Liver involvement | 6 (22%) |
| Median serum bilirubin (μmol/L) | 6 (2-50) |
| Median ALP (U/L) | 105 (25-1879) |
| Soft tissue involvement | 2 (7%) |
| Peripheral nerve involvement | 6 (22%) |
| Autonomic nerve involvement | 4 (15%) |
| Lymph node involvement | 13 (48%) |
| GI involvement | 0 |
| Median number of involved organs | 1 (1-4) |
| Involved light chains | |
| κ | 7 (26%) |
| Λ | 12 (44%) |
| No monoclonal light-chain excess | 8 (30%) |
| Median dFLC (mg/L) | 59.8 (2.2-856.4) |
| IgM κ M-protein, IgM λ M-protein | 11 (44%), 14 (56%) |
| Median serum monoclonal protein (g/L) | 11.5 (1-30) |
ALP, alkaline phosphatase; dFLC, difference between involved and uninvolved light chains; ECOG, Eastern Cooperative Oncology Group; GFR, glomerular filtration rate; GI, gastrointestinal; LV, left ventricular; NT-pro-BNP, N-terminal pro–brain natriuretic peptide; NYHA, New York Heart Association.
Twenty-one patients had available bone marrow data (trephine with or without flow cytometry; 3 normal, 1 plasma cell infiltration, 14 lymphoplasmacytic lymphoma, and 3 NHL not specifically classified). Five patients were treated with BR for refractory disease; previous therapies included bortezomib-cyclophosphamide-dexamethasone in 2 patients and rituximab-bortezomib-dexamethasone, rituximab-cyclophosphamide-vincristine-prednisolone, and rituximab-cyclophosphamide-dexamethasone in 1 case each.
The median number of BR cycles was 5 (range, 1-8). The median number of previous cycles in the second-line group was 6 (range, 4-8). Three patients received twice-monthly rituximab maintenance (375 mg/m2 IV) after first-line therapy with BR. All 3 patients remain on rituximab maintenance, with treatment duration thus far of 8, 10, and 11 months.
ORR on an intention-to-treat (ITT) and evaluable basis was 59% and 76%, respectively. Hematological responses (ITT) were a complete response (CR) in 11% of patients, VGPR in 37%, partial response (PR) in 11%, and no response (NR) in 41% (including 22% deaths). In the first-line group, hematological responses were CR 14%, VGPR 32%, PR 14%, and NR 40% (including death in 18% of patients). The evaluable responses were CR 17%, VGPR 39%, PR 17%, and NR 27%. Of the 3 patients treated with twice-monthly rituximab maintenance, 1 remains in VGPR, 1 in CR (having been in VGPR at time of completing BR), and 1 in PR (nonresponder at time of BR completion). Among the 5 patients treated for refractory AL, 3 achieved a VGPR and 2 were nonresponders (including 1 death). Three out of 17 patients with cardiac involvement achieved N-terminal pro–brain natriuretic peptide responses, and 3 out of 17 patients achieved renal responses by consensus criteria.10
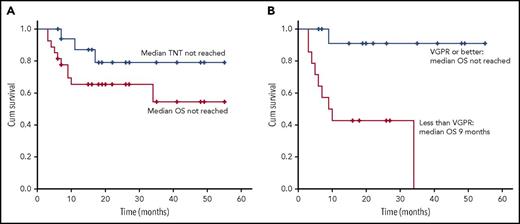
Median follow-up was 18 months (3-55 months). Median OS was not reached (Figure 1A). Median OS was not reached in patients who achieved VGPR or better (compared with 9 months in patients who did not) (Figure 1B). Median PFS was 34 months. Five patients (18.5%) died within 6 months of diagnosis (3 Mayo stage II and 2 stage III). On 6-month landmark analysis, median PFS was not reached. Of patients in the 6-month landmark analysis who achieved VGPR or better, median OS and PFS were not reached (compared with 34 and 11 months, respectively, in patients who did not). Three patients progressed to next treatment (their response to BR was VGPR, PR, and NR). Median TNT was not reached, with 88% and 79% of evaluable patients (n = 17) not progressing to next treatment at 1 and 3 years, respectively (Figure 1A).
OS and TNT. (A) OS on an ITT basis (n = 27) and TNT on an evaluable basis (n = 17). Median OS and median TNT were not reached. OS at 1 and 3 years was 65% and 56%, respectively. At 1 and 3 years, 88% and 79% of evaluable patients (n = 17) had not progressed to further treatment. (B) Median OS was not reached in those patients who achieved a VGPR or better, with 92% alive at 2 years; median OS was 9 months in patients who did not achieve a VGPR. Cum survival, cumulative survival.
OS and TNT. (A) OS on an ITT basis (n = 27) and TNT on an evaluable basis (n = 17). Median OS and median TNT were not reached. OS at 1 and 3 years was 65% and 56%, respectively. At 1 and 3 years, 88% and 79% of evaluable patients (n = 17) had not progressed to further treatment. (B) Median OS was not reached in those patients who achieved a VGPR or better, with 92% alive at 2 years; median OS was 9 months in patients who did not achieve a VGPR. Cum survival, cumulative survival.
Grade 3-4 toxicity included diarrhea 1 (3.7%), hypotension 2 (7.4%), nonneutropenic infection (11.1%), neutropenia 2 (7.4%), febrile neutropenia 2 (7.4%) (resulting in bendamustine dose reduction to 60 mg/m2), rash 1 (3.7%), fluid overload 2 (7.4%), and gastrointestinal bleeding 1 (3.7%). The most frequent grade 1-2 toxicities were constipation (26%) and fatigue (30%).
The data from this small retrospective study demonstrate excellent hematological responses. Impressively, 48% achieved VGPR or better (ITT). Sixty percent of patients treated with second-line BR achieved a VGPR. Furthermore, rituximab maintenance upgraded responses in the few patients that received this therapy.
Treatment in IgM-AL is not uniform, with poor responses to alkylators (responses 27% to 38%2,11,12 ) or purine-analog/anthracycline–containing therapy.1,2 Outcomes with bortezomib-based therapy are mixed (VGPR or better of 27% and 42% in 2 studies).1,13 A rituximab-bortezomib combination improved responses (ORR, 78%).3 The high prevalence of disease-related neuropathy in IgM-AL provokes reticence in using bortezomib. Furthermore, bortezomib is not funded in the United Kingdom for lymphoproliferative disorders. The few patients eligible for autologous stem cell transplantation appear to have good outcomes (ORR, 89% and 67% organ responses) but transplant-related mortality was 8%.4 In a large European study, only 1.8% of patients were treated with autologous stem cell transplantation.1 Disappointingly, the efficacy of ibrutinib in Waldenstrom macroglobulinemia (ORR, 91%)14 was not replicated in AL.5
In conclusion, this study suggests that first-line BR leads to high response rates in IgM-AL. Bendamustine is not neurotoxic, dosing is not affected by renal impairment, and there is no known cardiac toxicity, rendering BR a widely applicable therapy in IgM-AL. Response appears durable, and maintenance rituximab may upgrade depth of response. The limitation of this study is one shared with almost all literature in IgM-AL given its rarity: it is small and retrospective. Larger collaborative studies are needed to confirm these results, and combination with novel proteasome inhibitors should be explored to further improve outcomes.
Acknowledgments
The authors acknowledge their staff in echocardiography and histopathology and those involved in the clinical care of their patients. The alchemy study was performed in accordance with the recommendations guiding physicians in biomedical research involving human subjects adopted by the 18th World Medical Assembly, Helsinki, Finland, 1964, amended at the 52nd World Medical Association General Assembly, Edinburgh, Scotland, 2000. Informed written consent was obtained from the patients prior to registration into the study. The right of a patient to refuse participation without giving reasons is respected. Patients remain free to withdraw at any time from the study without giving reasons and without prejudicing his/her further treatment. Approval for this study was granted by the appropriate United Kingdom National Health Service Research Ethics Committee.
Authorship
Contribution: R.M. and A.D.W. designed the study, collected and analyzed data, and wrote the paper; and S.S., S.M., D.F., F.S., T.R., T.L., C.Q., M.F., H.J.L., J.D.G., C.W., and P.N.H. managed patients and provided critical input prior to submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests
Correspondence: Ashutosh Wechalekar, National Amyloidosis Centre, University College London (Royal Free Campus), Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: a.wechalekar@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal