TO THE EDITOR:
Alibert first described mycosis fungoides (MF) in 1806, and Bazin later defined the natural evolution into the stages known today as patch, plaque, and tumor.1 In the earliest stage, known as IA, MF is indolent. As progression ensues, extracutaneous invasion is not uncommon; however, there is minimal literature describing intraocular involvement. Since 1957 when German physician J. Gärtner first described intraocular MF, fewer than 10 cases have been published.2-11 Intraocular disease appears to portend a poor prognosis, and predicting which patients may exhibit this phenotype is of great interest, as early screening and treatment may prevent vision loss. Importantly, 1 report of intraocular MF detailed an immunophenotypic shift from an early skin biopsy taken at diagnosis to a vitreous biopsy taken several years later.8 In this letter, we herein discuss 2 patients with stage IA MF who underwent an immunophenotypic switch in the skin, prior to and concomitantly with, the development of intraocular involvement. This may help shed some light on the role of immunophenotype in disease progression and aggressiveness.
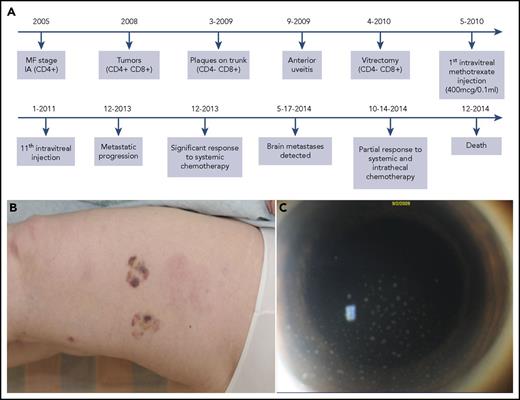
In 2005, a 54-year-old woman was diagnosed with stage IA MF and initially managed with narrowband UVB phototherapy (Figure 1A). As her disease progressed over several years, serial skin biopsies demonstrated a curious immunophenotypic shift (Figure 1B). In 2008, right eye visual acuity rapidly declined. Vitreous biopsy with flow cytometry confirmed intraocular MF (Figure 1C). Her vision and intraocular disease burden responded to a series of intravitreal methotrexate injections. Unfortunately, despite radiation and several systemic treatments, including cyclophosphamide, etoposide, vincristine, and prednisone, she developed a central nervous system (CNS) relapse. In 2014, the patient succumbed to systemic dissemination of MF.
Patient 1 timeline, clinical progression, and intraocular disease burden. (A) Timeline of case 1, including the immunophenotype of the various tumor sites and the intraocular involvement. This timeline highlights the evolution of the immunophenotypic switch, the diagnosis of intraocular involvement, and the subsequent treatments. (B) The new cutaneous tumors, which erupted in 2008, were the first evidence of immunophenotypic switch from CD4+ to mixed CD4+/CD8+. (C) Anterior segment photograph demonstrating anterior chamber cellular reaction with keratic precipitates composed of tumor cells on the endothelial surface of the cornea. These intraocular cells were CD4−/CD8+.
Patient 1 timeline, clinical progression, and intraocular disease burden. (A) Timeline of case 1, including the immunophenotype of the various tumor sites and the intraocular involvement. This timeline highlights the evolution of the immunophenotypic switch, the diagnosis of intraocular involvement, and the subsequent treatments. (B) The new cutaneous tumors, which erupted in 2008, were the first evidence of immunophenotypic switch from CD4+ to mixed CD4+/CD8+. (C) Anterior segment photograph demonstrating anterior chamber cellular reaction with keratic precipitates composed of tumor cells on the endothelial surface of the cornea. These intraocular cells were CD4−/CD8+.
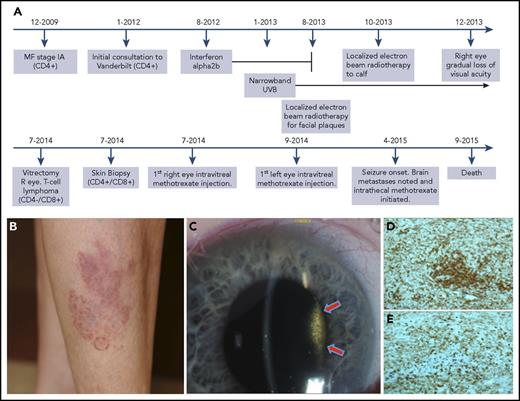
Patient 2, a 69-year-old woman diagnosed with stage IA MF in 2009, was initially managed with narrowband UVB phototherapy and topical tazarotene (Figure 2A). Given progression, she was subsequently managed with oral bexarotene, interferon α2b, and local electron beam radiotherapy (Figure 2A). In 2013, her bilateral visual acuity declined, and ophthalmologic examinations showed vitritis in both eyes. A vitrectomy and flow cytometry of aqueous fluid confirmed intraocular MF. Skin biopsies at this time also demonstrated an immunophenotypic shift. Intravitreal methotrexate afforded return of visual acuity. In April 2015, she developed CNS dissemination, and despite treatment with prednisone and intrathecal methotrexate, she succumbed to her disease.
Patient 2 timeline, clinical progression, vitreous tumor, and immunohistochemical stains demonstrating shift. (A) Timeline of case 2, including the evolution of the immunophenotypic switch, the diagnosis of intraocular involvement, and the treatment course. (B) An ill-defined red plaque with multiple follicular-based red papules present during initial consultation. (C) Slit lamp photograph of the right eye showing dense vitreous tumor cellular involvement behind the lens. The vitritis filled the entire vitreous cavity, but is only visible in this photograph in the area illuminated by the slit beam (arrows). (D-E) Immunohistochemical stains of cutaneous biopsy taken at time of intraocular diagnosis. Note the strong positivity for both CD4 (D) and CD8 (E) indicating a mixed immunophenotype of the cutaneous tissue, even at the same time that the intraocular tumor was CD4−/CD8+. Original magnification ×10 for panels D-E. R, right.
Patient 2 timeline, clinical progression, vitreous tumor, and immunohistochemical stains demonstrating shift. (A) Timeline of case 2, including the evolution of the immunophenotypic switch, the diagnosis of intraocular involvement, and the treatment course. (B) An ill-defined red plaque with multiple follicular-based red papules present during initial consultation. (C) Slit lamp photograph of the right eye showing dense vitreous tumor cellular involvement behind the lens. The vitritis filled the entire vitreous cavity, but is only visible in this photograph in the area illuminated by the slit beam (arrows). (D-E) Immunohistochemical stains of cutaneous biopsy taken at time of intraocular diagnosis. Note the strong positivity for both CD4 (D) and CD8 (E) indicating a mixed immunophenotype of the cutaneous tissue, even at the same time that the intraocular tumor was CD4−/CD8+. Original magnification ×10 for panels D-E. R, right.
In case 1, initial diagnostic skin biopsies demonstrated typical CD4+CD8− MF. Biopsies of tumors in 2008 showed mixed CD4+CD8+ disease (Figure 1B). In 2009, the immunophenotype deviated further to CD4−CD8+, with aberrant CD19 positivity. Subsequent vitreous biopsy and flow cytometry verified CD4−CD8+ MF with aberrant CD19, compatible with earlier skin biopsies. An identical clone, confirmed with T-cell receptor–γ gene rearrangement, was seen in the tissue from 2008, 2009, and the vitreous. The clone was similar, but not identical to original skin from 2005.
In case 2, skin biopsies from 2012 demonstrated CD4+CD8− MF. In 2014, flow cytometry of the vitreous specimen revealed CD4−CD8+CD56+ T cells confirming intraocular MF. Two days later, skin biopsies also demonstrated an immunophenotypic shift to CD4+CD8+ (Figure 2D-E). A subsequent skin biopsy in 2014 demonstrated a further derangement to CD4+/−CD8+CD56+. T-cell receptor–γ gene rearrangement demonstrated a distinctive clone from biopsies taken from 2012.
A similar treatment approach was taken for both patients. Intravitreal methotrexate (400 μg/0.1 mL) was initiated weekly for 4 weeks and then tapered to every other week and eventually monthly. Patient 1 received 11 total injections. Her vision improved from 20/150 at induction to 20/20 at completion with no evidence of intraocular disease. Patient 2 received 18 total injections. Disease burden improved from initial 4+ vitreous cells in the right eye and 3+ vitreous cells in the left to complete resolution. Visual acuity improved from hand motions in the right eye and 20/20 in the left to 20/20 bilaterally.
Ocular and periocular involvement is estimated to occur in ∼2% of MF patients, with the eyelid being most commonly affected.12 True intraocular involvement is very rare, with the vitreous most commonly involved in reported cases.3,4,6-8,10-12 Intraocular involvement seems to herald a poor prognosis with 10 of the 12 reported cases in the literature succumbing to advanced disease,2-5,7,8,11 whereas the remaining 2 were lost to follow-up.6,9 Thus, including our 2 cases, no documented patient with intraocular MF has survived. Moreover, all previous reports indicate death occurring between 17 days and 1 year following intraocular diagnosis.2-9,11 Our patients survived 58 months and 14 months after intraocular involvement was diagnosed, which likely attests to advances in therapies and lack of other extracutaneous invasion. Notably, only 2 reports indicate clear onset of intraocular disease preceding additional metastases,3,6 because the majority harbor widespread disease, including concomitant CNS seeding, at the time of intraocular involvement.2,4,5,7-9,11
This raises the following question: how can we predict who is at risk for intraocular involvement, so that screening and early intervention can be instituted in these high-risk patients?
CD8+ MF, representing <5% of cases,13,14 seems to parallel CD4+ disease15,16 ; however, although no long-term studies have confirmed this hypothesis, an immunophenotypic CD4+ to CD8+ switch appears to be emerging as a poor prognostic indicator.8,17-19 Prior to our report, only 1 previous case8 of intraocular MF documented the immunophenotypic characteristics of a vitreous sample. In this report, and our 2 patients, a divergence from original skin biopsies was discovered, with evolution from CD4+ to CD8+ predominance. We propose that if evolution from CD4+ to CD8+ predominance occurs in skin biopsies, clinical suspicion for the prospect of intraocular disease should be aroused and ophthalmology consulted, even in the absence of known extracutaneous disease. In cases of confirmed intraocular MF, we have had success using intensive intravitreal methotrexate injections, with dramatic improvements in intraocular disease burden and visual function. Thus, in such cases, referral to a specialty ocular oncology center may be warranted. Finally, close monitoring for further aggressive disease, especially CNS involvement, becomes vital.
Presented in abstract form at the European Organization of Research and Treatment of Cancer Cutaneous Lymphoma Task Force meeting, Paris, France, 26 October 2014.
Acknowledgments
This work is funded by National Institutes of Health, National Eye Institute grant 1K08EY027464-01 and by a Career Development Award from Research to Prevent Blindness (A.B.D.).
Authorship
Contribution: J.A.Z. was the lead physician managing MF in both patients; J.P.Z. was the corroborating dermatopathologist in both cases; S.J.K. was the lead ophthalmologist for case 1 and A.B.D. was the lead ophthalmologist for case 2; J.A.B. was first author in charge of data collection, literature review, and writing; and all authors contributed equally to editing this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan A. Braue, Vanderbilt Dermatology, 719 Thompson Ln, Suite 26300, Nashville, TN 37204; e-mail: jonathan.a.braue@vanderbilt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal