Key Points
Allo-HSCT with RIC is safe and effective in younger adults with severe PID.
Referral triggers should include severe infections, autoimmunity, malignancy, and disease progression despite conservative management.
Abstract
The primary immunodeficiencies (PIDs), rare inherited diseases characterized by severe dysfunction of immunity, have been successfully treated by allogeneic hematopoietic stem cell transplantation (Allo-HSCT) in childhood. Controversy exists regarding optimal timing and use of Allo-HSCT in adults, due to lack of experience and previous poor outcomes. Twenty-nine consecutive adult patients, with a mean age at transplant of 24 years (range, 17-50 years), underwent Allo-HSCT. Reduced-intensity conditioning (RIC) included fludarabine (Flu)/melphalan/alemtuzumab (n = 20), Flu/busulfan (Bu)/alemtuzumab (n = 8), and Flu/Bu/antithymocyte globulin (n = 1). Stem cell donors were matched unrelated donors or mismatched unrelated donors (n = 18) and matched related donors (n = 11). Overall survival (OS), event-free survival, transplant-related mortality (TRM), acute and chronic graft-versus-host disease incidence and severity, time to engraftment, lineage-specific chimerism, immune reconstitution, and discontinuation of immunoglobulin replacement therapy were recorded. OS at 3 years for the whole cohort was 85.2%. The rarer PID patients without chronic granulomatous disease (CGD) achieved an OS at 3 years of 88.9% (n = 18), compared with 81.8% for CGD patients (n = 11). TRM was low with only 4 deaths observed at a median follow-up of 3.5 years. There were no cases of early or late rejection. In all surviving patients, either stable mixed chimerism or full donor chimerism were observed. At last follow-up, 87% of the surviving patients had no evidence of persistent or recurrent infections. Allo-HSCT is safe and effective in young adult patients with severe PID and should be considered the treatment of choice where an appropriate donor is available.
Introduction
The primary immunodeficiencies (PIDs) are a rare group of inherited diseases characterized by severe dysfunction of adaptive and/or innate immunity, typically arising from genetic mutations in hematopoietic stem cells. Nearly 300 distinct immunodeficiencies have now been described, with 20 accounting for over 90% of cases. Excluding common variable immunodeficiency (CVID), 3 of the most common are severe combined immunodeficiency (SCID), Wiskott-Aldrich syndrome, and chronic granulomatous disease (CGD). Children with severe PIDs have been successfully treated by allogeneic hematopoietic stem cell transplantation (Allo-HSCT), which has been the major therapeutic option for inherited cellular immunodeficiency disorders since 1968.1-6 However, almost all series published on outcomes for Allo-HSCT have focused on pediatric patients. The median age at transplant of patients described in the largest published series is <1 year for SCID,7 2.8 years for Wiskott-Aldrich syndrome,8 and 12.7 years for CGD, using reduced-intensity conditioning (RIC).9,10
Significant advances in hematopoietic transplantation over the past 20 years, including refinement of HLA-tissue typing, adoption of RIC regimens, increased availability of alternative stem cell sources, improved methods of graft-versus-host disease (GVHD) prophylaxis, and improved supportive care, have translated into better outcomes today compared with the early experience.5,11
Early Allo-HSCT is important in infants or children presenting with serious or life-threatening infections because without definitive treatment, patients with severe PID, such as SCID, rarely survive beyond 1 year of age. In addition, younger patients have less end-organ damage from repeated or severe infections.11,12 Indeed, overall survival (OS) has been shown to fall from 95% in SCID patients transplanted at ages <3.5 months to 76% in older children.7,13
Although early Allo-HSCT is preferred for PID, this is often not possible. An initial milder clinical phenotype, delayed diagnosis, late presentation, lack of a genetic diagnosis, or an inability to identify a suitable stem cell donor may result in patients surviving to adolescence and early adulthood without having undergone Allo-HSCT. Furthermore, in non-SCID forms of PIDs, the clinical phenotype can be very heterogeneous and “atypical,” largely due to the high number of genetic and functional defects affecting T, B, and NK cells, neutrophils, and/or antigen presentation. For these patients, diagnosis can be delayed or difficult and the natural history of exceptionally rare underlying diseases is often unclear. In several PIDs, controversy surrounding optimum timing of Allo-HSCT remains, due to rarity of disease, lack of experience, emerging gene therapies,14-17 and infrequent published outcome data due to the very low numbers in any 1 center.
One such disorder where optimum timing remains unclear is CGD, which is characterized by impairment of the phagocyte reduced nicotinamide adenine dinucleotide phosphate (NADPH)–oxidase complex. Patients with absent NADPH activity are one group thought to benefit from early transplant18 and criteria have been devised for Allo-HSCT in children. These are: life-threatening infection, noncompliance with antimicrobial prophylaxis, or steroid-dependent autoinflammatory disease.19 In adolescents and adults, criteria are more difficult to apply due to higher rates of organ dysfunction and reported increased mortality from Allo-HSCT.20 The largest published case series reported a cohort of 70 CGD patients with excellent OS of 91.4% at median follow-up (34 months), but this cohort had a median age of 8.9 years (interquartile range [IQR], 3.8-19.3 years).10 Similarly, the second largest series of 56 CGD patients also had an excellent OS of 93% at median follow-up (21 months), but again the median age was young at 12.5 years9 with only 14 patients aged ≥17 years of age.
There is even less experience of Allo-HSCT in rarer PIDs and the literature is confined to pediatric series or adult case reports. In the lethal genetic PID caused by GATA2 mutations, HSCT has resulted in reversal of the hematologic, immunologic, and clinical phenotype in small series including adults.21,22 In addition, in X-linked inhibitor of apoptosis protein (XIAP) deficiency, poor outcomes post–Allo-HSCT have been reported, with high rates of transplant-related mortality (TRM),23 although a recent article has described excellent outcome for 10 Japanese patients (all <17 years) undergoing RIC-conditioned HSCT.24
Given the reduced toxicity of RIC regimens, these appear to be better tolerated in patients with PID who often have significant organ dysfunction and associated elevated hematopoietic cell transplant–comorbidity index (HCT-CI) scores25 pretransplant. Elsewhere, others have used low-toxicity myeloablative conditioning regimens incorporating targeted treosulfan with excellent results in children.26,27
We report here the outcome of 29 consecutive adult patients (≥17 years) who underwent Allo-HSCT for a variety of PIDs in the Allo-HSCT programs of University College London (UCL) Hospitals NHS Foundation Trust and the Royal Free London Hospital NHS Foundation Trust, UCL Centre for Immunodeficiency, July 2004 to November 2016. Prior to transplant, all patients had developed complications that necessitated definitive treatment with curative intent. During the study period, 5 additional adult patients were referred for consideration of Allo-HSCT who did not proceed to transplant. Referral was triggered by life-threatening infection, malignancy, autoimmune or inflammatory phenomena, new genetic diagnosis, or new donor availability.
Methods
Study design and participants
All patients provided written consent as per institutional practice for Allo-HSCT. The mean age at transplant was 24 years (range, 17-50 years). Of these, 11 patients had CGD and 18 had various other PIDs. Detailed patient demographics and transplant characteristics are shown in Table 1. PIDs were diagnosed using international criteria. Patients with severe dysfunction of adaptive and/or innate immunity can present with recurrent infections, autoimmunity, and/or malignancy. Nineteen patients had a genetic diagnosis and 10 had a combination of clinical phenotype with a corresponding functional defect. Secondary causes of immunodeficiency were excluded. Data on donor status, conditioning regimen, and clinical condition during and after transplant and at last follow-up was collected retrospectively from the medical notes and our Allo-HSCT database. The HCT-CI is a comorbidity tool, which captures the prevalence, magnitude, and severity of various organ impairments before Allo-HSCT to predict risk of TRM. HCT-CI scores pretransplant were calculated for all patients.22 All patients had a score of ≥1 whereas 12 (41%) had a score of ≥3. HCT-CI scores were higher in the PID cohort (50% scored ≥3) compared with the CGD cohort (27% scored ≥3), which was not statistically significant (P = .206), but may suggest an increased risk of TRM.
Patient demographics and transplant characteristics
| Pt . | Diagnosis . | Genetic mutation . | Age at diagnosis, y . | Age at HSCT/ sex . | Medical complications prior to transplant . | HCT-CI score* . | Donor (HLA mismatch) . | Stem cell source . | CD34 dose, ×106/kg . | CMV status (R/D) . | Conditioning regimen . | In vivo T-cell depletion (dose) . | GVHD prophylaxis . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CVID | Not done. | 20 | 31/F | Red cell aplasia. | 2 | MRD | PBSC | 10.8 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 2 | Granulomatous CVID | No mutation in TNFSF5. (Normal T-cell numbers at presentation and no T-cell related infections). | 15 | 30/F | Inflammatory lung disease, sclerosing cholangitis, prior splenectomy, pulmonary fibrosis, pulmonary aspergilloma,* osteopenia. | ≥3 | MUD | PBSC | 15.3 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 3 | APDS2 | Splice donor mutation in PIK3R1 (predicting exon skipping). | 10 | 50/M | Severe lymphatic pancolitis, previous mycoplasma arthritis, bronchiectasis, previous peripheral CD8+ T-cell NHL, pulmonary hypertension. | ≥3 | MUD | PBSC | 5.1 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 4 | Autoimmune LPD | Heterozygous TNFRSF 6 gene encoding Fas (c.785T>C (I232T). | 3 | 32/M | Refractory thrombocytopenia, previous splenectomy, hypertension. | 2 | MRD | PBSC | 3.7 | +/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 5 | Autoimmune LPD | No mutation identified, including in Fas, SH2D1A. Functional apoptosis defect. | 32 | 34/M | Indolent lymphoma (NHL), autoimmune neutropenia, MGUS with IgG paraprotein, previous splenectomy, factor XI deficiency. | 1 | MUD | PBSC | 6.7 | −/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 6 | Cγ-chain SCID | Confirmed by sequencing. | 1 | 26/M | Failed haploidentical allo and gene therapy. Sclerosing cholangitis. Recurrent infections. Bronchiectasis and pneumocele. Brachiocephalic thrombus. Hepatomegaly with chronic cholangiopathy. Refractory facial planar warts. Depression. | ≥3 | MUD | PBSC | 12.6 | +/+ | Flu Mel | Alemuzumab (100 mg) | CSA |
| 7 | Absolute NK deficiency, hypogammaglobulinemia | No mutation identified. Whole-genome sequencing pending. | 14 | 27/M | Refractory planar warts (HPV2),† recurrent infections, bronchiectasis, sarcoid arthropathy, IgG 1 and 2 subclass deficiency. | ≥3 | MRD | PBSC | 7.8 | +/+ | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 8 | DCML deficiency | Gata2 wild type; whole-exome sequencing. Pending. | 24 | 27/F | Trilineage MDS, bronchiectasis, Crohn colitis, recurrent viral warts, Mycobacterium avium complex, AIN and vulval intraepithelial neoplasia (HPV)†. | ≥3 | MMUD (C Ag) | PBSC | 6.3 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 9 | AR IL12Recβ deficiency | Confirmed by sequencing. | 19 | 29/M | Recurrent Salmonella sepsis, Salmonella pericarditis with cardiac tamponade, mild asthma, recurrent otitis externa. | ≥3 | MMUD (A Ag) | PBSC | 7.0 | −/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 10 | Rag2/red cell aplasia | RAG2 (c.104G>T (Gly35Val)/het and c.814G>A (Val72Ile)/het andc.965T>C(Met 322Thr)Het. | 16 | 20/M | Red cell aplasia, granulomatous skin lesions, high transfusion requirement and iron overload, chronic clonal NK-cell proliferation, EBV viremia. | ≥3 | MUD | PBSC | 5.4 | −/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 11 | X-linked LPD | Confirmed by sequencing. | 3 | 18/M | B-cell NHL, hypogammaglobulinemia. | 1 | MRD | PBSC | 11.0 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 12 | Undefined CID | c.205del heterozygote in CD27 gene. Heterozygous mutations in CD27, LRBA, LYST, and PRKDC. | 11 | 22/M | EBV viremia, atypical stage 4B T-cell rich B-cell NHL, LP HL (R-CHOP ×6, high dose MTX). | 2 | MUD | PBSC | 4.0 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 13 | CD27 deficiency | CD27 (c.251_252insT [C71Lfs*4] and SNP in exon 3 371761387). | 13 | 18/M | Nodular sclerosing HL, EBV+ stage IV diffuse large B-cell lymphoma. | 1 | MMUD (A Ag) | PBSC | 6.4 | −/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 14 | Gata2 deficiency | 735-736insC amino acid Subs P245fs. | 20 | 22/F | MDS, persistent viral warts, mild IgG hypogammaglobulinemia, CD19, CD56 lymphopenia, HPV VIN3†. | 1 | MUD | PBSC | 6.3 | +/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 15 | XIAP deficiency | XIAP: absent by FACs (2 centers); no mutation identified. | 19 | 21/M | HLH, Crohn (requiring pancolectomy as a child). | ≥3 | MRD | PBSC | 4.7 | −/+ | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 16 | Autoimmune LPD | I246T Fas mutation. | 2 | 21/F | Relapsed Hodgkin lymphoma, autoimmune hemolysis, genital HSV, previous splenectomy, juvenile inflammatory arthropathy, patchy colonic lymphocytic infiltration. | 2 | MRD | PBSC | 2.1 | +/+ | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 17 | Gata 2 mutation | Frame duplication of 6 nucleotides not previously reported. | 19 | 22/F | MDS with profound monocytopenia, prolonged severe EBV infection with hepatitis and meningoencephalitis. | ≥3 | MRD | PBSC | 2.0 | −/− | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 18 | XIAP deficiency | Sequence variant c.497G>A in exon 2 of the XIAP gene. Not previously reported. | 4 | 24/M | EBV lymphoproliferative disease ×2 episodes, hypogammaglobulinemia, splenomegaly, granulomatous lymphocytic inflammatory lung disease with bronchiectasis. | 2 | MRD | PBSC | 5.5 | +/− | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 19 | AR-CGD | P47 deficiency identified on FACs analysis. | 12 | 18/M | Recurrent infections including staphylococcal abscesses. | 1 | Father 10/10 | BM | 3.7 | +/+ | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 20 | AR-CGD | p47 deficiency identified on FACs analysis. | 18 | 19/M | Recurrent infections, chronic relapsing multifocal osteomyelitis, mold pulmonary infection, dyslexia. | 2 | MUD | BM | 2.1 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 21 | AR-CGD | NCF-1 (P47 deficiency by FACs. Homozygous for c.579g>A (Trp193X). | 3 | 27/F | Recurrent infections including presumed nocardia meningitis. Severe inflammatory bowel disease necessitating subtotal colectomy and ileostomy formation. Colonized with resistant Pseudomonas. | ≥3 | MUD | BM | 6.3 | +/+ | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 22 | Variant CGD | NCF-1 (fusion of NCF-1 and pseudo NCF-1 with crossover between exon 4 and exon 16). | 24 | 28/F | Severe Crohn disease with perineal and perianal disease requiring colectomy and proctectomy with ileostomy. | 1 | MUD | PBSC | 14.5 | −/− | Flu Bu | rATG (7.5 mg/kg) | CSA |
| 23 | X-linked CGD | CYBB (c.G764A[Trp251*]). | 1 | 27/M | Recurrent infections, severe colitis. | 2 | MRD | PBSC | 5.0 | +/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 24 | X-linked CGD | p47 deficiency identified on FACs analysis. | 2 | 18/M | Pancolitis, previous hemicolectomy for stricture of ascending colon, recurrent infections, renal impairment. | ≥3 | MUD | BM | 4.2 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 25 | X-linked CGD | CYBB (TC342/343 > AT [His115Tyr]). | 4 | 19/M | Recurrent fungal chest infections, extensive granulomas, growth failure, previous gene therapy (August 2007) with transient engraftment. | ≥3 | MMUD (A Ag) | PBSC | 32.3 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 26 | X-linked CGD | GP91 intron6 gtg>atg. | 1 | 19/M | Recurrent infections including osteomyelitis and fungal chest infection (Aspergillus nidulans). | 1 | MMUD (A Ag) | BM | 0.3 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 27 | X-linked CGD | GP91phox 20-kb deletion leading to complete absence of CYBB and Kell genes (McLeod phenotype). | At birth | 17/M | Pancolitis, recurrent infections, McLeod phenotype. | 2 | MUD | BM | 1.4 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 28 | X-linked CGD | NCF1 (c.579G>A (TRP193*). | 16 | 23/M | Recurrent infections including staphylococcal skin abscesses, progressive granulomas. | 2 | MRD | PBSC | 4.1 | +/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 29 | X-linked CGD | Gp91absent by FACs. | 2 mo | 17/M | Progressive colitis, multiple infective complications. | 2 | MUD | BM | 1.9 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| Pt . | Diagnosis . | Genetic mutation . | Age at diagnosis, y . | Age at HSCT/ sex . | Medical complications prior to transplant . | HCT-CI score* . | Donor (HLA mismatch) . | Stem cell source . | CD34 dose, ×106/kg . | CMV status (R/D) . | Conditioning regimen . | In vivo T-cell depletion (dose) . | GVHD prophylaxis . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CVID | Not done. | 20 | 31/F | Red cell aplasia. | 2 | MRD | PBSC | 10.8 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 2 | Granulomatous CVID | No mutation in TNFSF5. (Normal T-cell numbers at presentation and no T-cell related infections). | 15 | 30/F | Inflammatory lung disease, sclerosing cholangitis, prior splenectomy, pulmonary fibrosis, pulmonary aspergilloma,* osteopenia. | ≥3 | MUD | PBSC | 15.3 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 3 | APDS2 | Splice donor mutation in PIK3R1 (predicting exon skipping). | 10 | 50/M | Severe lymphatic pancolitis, previous mycoplasma arthritis, bronchiectasis, previous peripheral CD8+ T-cell NHL, pulmonary hypertension. | ≥3 | MUD | PBSC | 5.1 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 4 | Autoimmune LPD | Heterozygous TNFRSF 6 gene encoding Fas (c.785T>C (I232T). | 3 | 32/M | Refractory thrombocytopenia, previous splenectomy, hypertension. | 2 | MRD | PBSC | 3.7 | +/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 5 | Autoimmune LPD | No mutation identified, including in Fas, SH2D1A. Functional apoptosis defect. | 32 | 34/M | Indolent lymphoma (NHL), autoimmune neutropenia, MGUS with IgG paraprotein, previous splenectomy, factor XI deficiency. | 1 | MUD | PBSC | 6.7 | −/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 6 | Cγ-chain SCID | Confirmed by sequencing. | 1 | 26/M | Failed haploidentical allo and gene therapy. Sclerosing cholangitis. Recurrent infections. Bronchiectasis and pneumocele. Brachiocephalic thrombus. Hepatomegaly with chronic cholangiopathy. Refractory facial planar warts. Depression. | ≥3 | MUD | PBSC | 12.6 | +/+ | Flu Mel | Alemuzumab (100 mg) | CSA |
| 7 | Absolute NK deficiency, hypogammaglobulinemia | No mutation identified. Whole-genome sequencing pending. | 14 | 27/M | Refractory planar warts (HPV2),† recurrent infections, bronchiectasis, sarcoid arthropathy, IgG 1 and 2 subclass deficiency. | ≥3 | MRD | PBSC | 7.8 | +/+ | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 8 | DCML deficiency | Gata2 wild type; whole-exome sequencing. Pending. | 24 | 27/F | Trilineage MDS, bronchiectasis, Crohn colitis, recurrent viral warts, Mycobacterium avium complex, AIN and vulval intraepithelial neoplasia (HPV)†. | ≥3 | MMUD (C Ag) | PBSC | 6.3 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 9 | AR IL12Recβ deficiency | Confirmed by sequencing. | 19 | 29/M | Recurrent Salmonella sepsis, Salmonella pericarditis with cardiac tamponade, mild asthma, recurrent otitis externa. | ≥3 | MMUD (A Ag) | PBSC | 7.0 | −/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 10 | Rag2/red cell aplasia | RAG2 (c.104G>T (Gly35Val)/het and c.814G>A (Val72Ile)/het andc.965T>C(Met 322Thr)Het. | 16 | 20/M | Red cell aplasia, granulomatous skin lesions, high transfusion requirement and iron overload, chronic clonal NK-cell proliferation, EBV viremia. | ≥3 | MUD | PBSC | 5.4 | −/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 11 | X-linked LPD | Confirmed by sequencing. | 3 | 18/M | B-cell NHL, hypogammaglobulinemia. | 1 | MRD | PBSC | 11.0 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 12 | Undefined CID | c.205del heterozygote in CD27 gene. Heterozygous mutations in CD27, LRBA, LYST, and PRKDC. | 11 | 22/M | EBV viremia, atypical stage 4B T-cell rich B-cell NHL, LP HL (R-CHOP ×6, high dose MTX). | 2 | MUD | PBSC | 4.0 | +/+ | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 13 | CD27 deficiency | CD27 (c.251_252insT [C71Lfs*4] and SNP in exon 3 371761387). | 13 | 18/M | Nodular sclerosing HL, EBV+ stage IV diffuse large B-cell lymphoma. | 1 | MMUD (A Ag) | PBSC | 6.4 | −/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 14 | Gata2 deficiency | 735-736insC amino acid Subs P245fs. | 20 | 22/F | MDS, persistent viral warts, mild IgG hypogammaglobulinemia, CD19, CD56 lymphopenia, HPV VIN3†. | 1 | MUD | PBSC | 6.3 | +/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 15 | XIAP deficiency | XIAP: absent by FACs (2 centers); no mutation identified. | 19 | 21/M | HLH, Crohn (requiring pancolectomy as a child). | ≥3 | MRD | PBSC | 4.7 | −/+ | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 16 | Autoimmune LPD | I246T Fas mutation. | 2 | 21/F | Relapsed Hodgkin lymphoma, autoimmune hemolysis, genital HSV, previous splenectomy, juvenile inflammatory arthropathy, patchy colonic lymphocytic infiltration. | 2 | MRD | PBSC | 2.1 | +/+ | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 17 | Gata 2 mutation | Frame duplication of 6 nucleotides not previously reported. | 19 | 22/F | MDS with profound monocytopenia, prolonged severe EBV infection with hepatitis and meningoencephalitis. | ≥3 | MRD | PBSC | 2.0 | −/− | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 18 | XIAP deficiency | Sequence variant c.497G>A in exon 2 of the XIAP gene. Not previously reported. | 4 | 24/M | EBV lymphoproliferative disease ×2 episodes, hypogammaglobulinemia, splenomegaly, granulomatous lymphocytic inflammatory lung disease with bronchiectasis. | 2 | MRD | PBSC | 5.5 | +/− | Flu Mel | Alemtuzumab (30 mg) | CSA |
| 19 | AR-CGD | P47 deficiency identified on FACs analysis. | 12 | 18/M | Recurrent infections including staphylococcal abscesses. | 1 | Father 10/10 | BM | 3.7 | +/+ | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 20 | AR-CGD | p47 deficiency identified on FACs analysis. | 18 | 19/M | Recurrent infections, chronic relapsing multifocal osteomyelitis, mold pulmonary infection, dyslexia. | 2 | MUD | BM | 2.1 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 21 | AR-CGD | NCF-1 (P47 deficiency by FACs. Homozygous for c.579g>A (Trp193X). | 3 | 27/F | Recurrent infections including presumed nocardia meningitis. Severe inflammatory bowel disease necessitating subtotal colectomy and ileostomy formation. Colonized with resistant Pseudomonas. | ≥3 | MUD | BM | 6.3 | +/+ | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 22 | Variant CGD | NCF-1 (fusion of NCF-1 and pseudo NCF-1 with crossover between exon 4 and exon 16). | 24 | 28/F | Severe Crohn disease with perineal and perianal disease requiring colectomy and proctectomy with ileostomy. | 1 | MUD | PBSC | 14.5 | −/− | Flu Bu | rATG (7.5 mg/kg) | CSA |
| 23 | X-linked CGD | CYBB (c.G764A[Trp251*]). | 1 | 27/M | Recurrent infections, severe colitis. | 2 | MRD | PBSC | 5.0 | +/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 24 | X-linked CGD | p47 deficiency identified on FACs analysis. | 2 | 18/M | Pancolitis, previous hemicolectomy for stricture of ascending colon, recurrent infections, renal impairment. | ≥3 | MUD | BM | 4.2 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 25 | X-linked CGD | CYBB (TC342/343 > AT [His115Tyr]). | 4 | 19/M | Recurrent fungal chest infections, extensive granulomas, growth failure, previous gene therapy (August 2007) with transient engraftment. | ≥3 | MMUD (A Ag) | PBSC | 32.3 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 26 | X-linked CGD | GP91 intron6 gtg>atg. | 1 | 19/M | Recurrent infections including osteomyelitis and fungal chest infection (Aspergillus nidulans). | 1 | MMUD (A Ag) | BM | 0.3 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 27 | X-linked CGD | GP91phox 20-kb deletion leading to complete absence of CYBB and Kell genes (McLeod phenotype). | At birth | 17/M | Pancolitis, recurrent infections, McLeod phenotype. | 2 | MUD | BM | 1.4 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
| 28 | X-linked CGD | NCF1 (c.579G>A (TRP193*). | 16 | 23/M | Recurrent infections including staphylococcal skin abscesses, progressive granulomas. | 2 | MRD | PBSC | 4.1 | +/− | Flu Mel | Alemtuzumab (100 mg) | CSA |
| 29 | X-linked CGD | Gp91absent by FACs. | 2 mo | 17/M | Progressive colitis, multiple infective complications. | 2 | MUD | BM | 1.9 | −/− | Flu Bu | Alemtuzumab (0.6 mg/kg) | CSA |
Ag, antigen; AIN, anal intraepithelial neoplasia; APDS2, activated phosphatidylinositol 3-kinase δ syndrome type 2; AR, autosomal recessive; BM, bone marrow; Bu, busulfan; CGD, chronic granulomatous disease; CID, combined immunodeficiency; CSA, cyclosporin A; CVID, common variable immunodeficiency; DCML, dendritic cell, monocyte, B lymphocyte, and natural killer lymphocyte deficiency; F, female; FACs, fluorescence-activated cell sorting; Flu, fludarabine; HCT-CI, hematopoietic cell transplant–comorbidity index; HL, Hodgkin lymphoma; HLH, hemophagocytic lymphohistiocystosis; HPV, human papilloma virus; HSV, herpes simplex virus; IgG, immunoglobulin G; LP HL, lymphocyte predominant HL; LPD, lymphoproliferative disease; M, male; MDS, myelodysplastic syndrome; Mel, melphalan; MGUS, monoclonal gammopathy of undetermined significance; MMUD, mismatched unrelated donor; MRD, matched related donor; MTX, methotrexate; MUD, matched unrelated donor; NHL, non-Hodgkin lymphoma; NK, natural killer cell; PBSC, peripheral blood stem cell; Pt, patient; rATG, rabbit antithymocyte globulin; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R/D, recipient/donor cytomegalovirus serostatus; SCID, severe combined immunodeficiency; SNP, single-nucleotide polymorphism; XIAP, X-linked inhibitor of apoptosis protein deficiency.
Active infection at transplant.
Unresolved viral warts at transplant.
Stem cell source
Eleven patients had matched related donors (MRDs; 10 siblings, one 10 of 10 matched paternal donor) and 18 had matched unrelated donors (MUDs), including 5 with 1 antigen mismatched unrelated donor (MMUD; 4 A antigen mismatch, 1 C antigen mismatch). Patients and donors were matched for HLA-A, -B, -C, -DRB1, and -DQB1 by intermediate or high-resolution DNA typing as appropriate.
Mobilized peripheral blood stem cells (PBSCs) were the stem cell source in 79% of the patients (n = 23, including all non-CGD patients) whereas bone marrow was used in the other 21% (n = 7, all CGD patients).
Allo-HSCT conditioning regimen
All patients were transplanted using previously described T-cell–depleted (in vivo alemtuzumab or antithymocyte globulin [ATG]) RIC regimens.9,28,29 All non-CGD PID patients received fludarabine (30 mg/m2 daily for 5 days), melphalan 140 mg/m2 and alemtuzumab (100 mg for MUD recipients delivered as 20 mg once daily, days −7 to day −3 and 30 mg single dose on day −1 for MRD recipients after May 2010). Two of the early MRD patients also received 100 mg of alemtuzumab delivered as 20 mg once daily, days −7 to day −3. Conditioning for CGD patients consisted of fludarabine (30 mg/m2 daily for 5 days), busulfan (1.6 mg/kg twice daily for 3 days), or melphalan (140 mg/m2 total), together with alemtuzumab (0.2 mg/kg daily for 3 days) for 10 patients or, in 1 patient, rabbit ATG (2.5 mg/kg daily for 3 days). The mean busulfan cumulative area under the curve was 56.05 μmol/L per minute (range, 44.34-62.66; n = 7). Details are shown in Table 1.
Supportive care
Reverse isolation, and antimicrobial and antifungal prophylaxis, were used to reduce the risk of infectious complications. All patients received prophylaxis against Pneumocystis jirovecii and acyclovir prophylaxis against varicella zoster virus reactivation. Surveillance for cytomegalovirus (CMV), adenovirus, and Epstein Barr virus (EBV) infection was performed by weekly polymerase chain reaction or antigenemia testing, and preemptive treatment was administered according to institutional guidelines.
In patients who were receiving immunoglobulin replacement therapy prior to transplant, this was continued until normal trough levels were demonstrated posttransplant and the risk of further infectious complications was minimal.
Chimerism analysis
Chimerism samples were processed at 3-monthly intervals until 1 year posttransplant and then 6 monthly or when indicated clinically (cytopenias, persistent infections, ongoing mixed chimerism). In patients with a sex-mismatched donor, chimerism was analyzed by fluorescence in situ hybridization. In those with sex-matched donors, polymerase chain reaction of short tandem repeats was used. Lineage-specific chimerism was performed on peripheral blood mononuclear cells (PBMCs) and T-cell, B-cell, and granulocyte compartments. The laboratory reports results as follows: “donor” where donor DNA was ≥97%, “mixed” where donor DNA was ≥50% and <97%, “very mixed” where there is more recipient than donor DNA (donor DNA ≥1% <50%), and “recipient” where no donor DNA can be detected.
Study end points and statistical analysis
Data were analyzed in April 2017, in accordance with published guidelines.32,33 Primary outcome measures were OS, event-free survival (EFS), TRM, neutrophil engraftment, and platelet engraftment. OS was defined as the time from transplant to death from any cause. EFS was defined as the time from transplant to graft failure, graft rejection, or death from any cause. Probabilities of OS and EFS were calculated using the Kaplan-Meier method with SPSS 22.0 statistical package (Chicago, IL). Comparison of survival curves was made using the log-rank method.
Cumulative incidence estimates were used to calculate TRM, and engraftment of neutrophils (≥0.5 × 109/L) and platelets (≥50 × 109/L).
Results
Survival
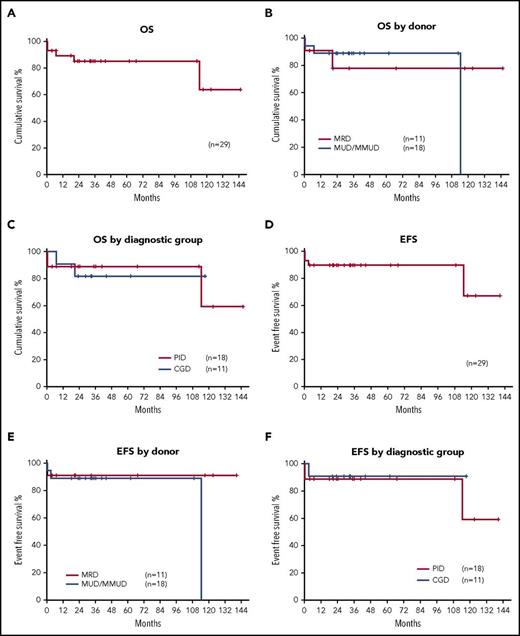
OS of the whole cohort was 89.2% at 1 year and 85.2% at 3 years, with a mean follow-up of 3.5 years (range, 4 months to 12 years). OS at 1 year was 90.9% and 77.9% at 3 years for MRD transplants (n = 11) and 94.4% at 1 year and 88.9% at 3 years for M/MUD transplants (P = .51; n = 18). OS was 90.9% at 1 year and 81.8% at 3 years for CGD patients (n = 11) and 94.4% at 1 year and 88.9% at 3 years for other PIDs (P = .75; n = 18) (Figure 1A-C).
Probabilities of OS and EFS. (A) OS for the whole cohort was 89.2% at 12 months and 85.2% at 3 years. (B) OS for MRDs 90.9% and 77.9% at 1 and 3 years, respectively. OS for MUD/MMUD transplants was 94.4% and 88.9% at 1 and 3 years, respectively (P = .51). (C) OS for PID patients was 94.4% and 88.9% at 1 and 3 years, respectively. OS for adults for CGD patients was 90.9% and 81.8% at 1 and 3 years (P = .75). (D) EFS was 89.7% at 1 and 3 years. (E) EFS for MRD transplants was 90.9% at both 1 and 3 years. EFS MUD/MMUD transplants was 88.9% at 1 and 3 years (P = .73). (F) EFS for PID patients was 88.9%; EFS for CGD patients was 90.9% at both 1 and 3 years (P = .65).
Probabilities of OS and EFS. (A) OS for the whole cohort was 89.2% at 12 months and 85.2% at 3 years. (B) OS for MRDs 90.9% and 77.9% at 1 and 3 years, respectively. OS for MUD/MMUD transplants was 94.4% and 88.9% at 1 and 3 years, respectively (P = .51). (C) OS for PID patients was 94.4% and 88.9% at 1 and 3 years, respectively. OS for adults for CGD patients was 90.9% and 81.8% at 1 and 3 years (P = .75). (D) EFS was 89.7% at 1 and 3 years. (E) EFS for MRD transplants was 90.9% at both 1 and 3 years. EFS MUD/MMUD transplants was 88.9% at 1 and 3 years (P = .73). (F) EFS for PID patients was 88.9%; EFS for CGD patients was 90.9% at both 1 and 3 years (P = .65).
EFS for the whole cohort was 89.7% at 1 and 3 years (n = 29), with no significant difference observed for EFS between MRD transplants and M/MUD transplants; 90.9% vs 88.9%, respectively (P = .73). There was no significant difference in EFS in patients with CGD and other PIDs: 90.9% vs 88.9% at 1 and 3 years (P = .65) (Figure 1D-F). One late death occurred in a patient over 9 years posttransplant due to respiratory complications in the context of progression of preexisting bronchiectasis.
Engraftment
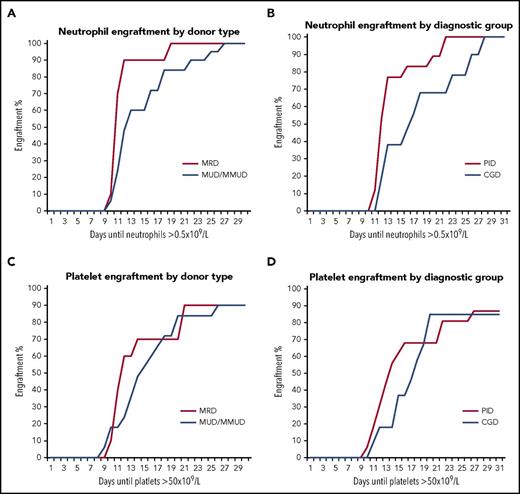
There were no episodes of graft rejection or graft failure. For the whole cohort, neutrophil engraftment occurred after a median 12 days (mean, 14 days; IQR, 11-17 days) and median platelet engraftment was 14 days (mean, 17 days; IQR, 11-20 days). Comparing MRD to MUD/MMUD, median time to neutrophil engraftment was 11 days (IQR, 11-12 days) vs 15 days (IQR, 12-21 days), respectively (Figure 2A). Comparing the 2 diagnostic groups, time to neutrophil engraftment was longer in patients with CGD (n = 11): median 16 days (IQR, 12-22 days) vs 11 days (IQR, 11-14 days) in patients with other PIDs (n = 18) (Figure 2B). The observed longer time to engraftment in the CGD group reflects the fact that 7 of 11 CGD patients had bone marrow as the stem cell source compared with the non-CGD PID patients who all received PBSCs.
Hematopoietic engraftment kinetics. (A) Cumulative incidence of neutrophil engraftment (defined as >0.5 × 109/L for 3 consecutive days). Median time to neutrophil engraftment was 11 days in MRD transplants and 15 days in MUD/MMUD transplants. (B) Cumulative incidence of neutrophil engraftment in the 2 main diagnostic groups. Median time to neutrophil engraftment was 11 days in non-CGD PIDs and 16 days in CGD patients. (C) Cumulative incidence of platelet engraftment (defined as >50 × 109/L for 3 consecutive days). Median time to platelet engraftment was 12 days in MRD transplants and 18 days in MUD/MMUD transplants. (D) Cumulative incidence of platelet engraftment in the 2 main diagnostic groups. Median time to platelet engraftment was 16 days in non-CGD PIDs and 19 days in CGD patients.
Hematopoietic engraftment kinetics. (A) Cumulative incidence of neutrophil engraftment (defined as >0.5 × 109/L for 3 consecutive days). Median time to neutrophil engraftment was 11 days in MRD transplants and 15 days in MUD/MMUD transplants. (B) Cumulative incidence of neutrophil engraftment in the 2 main diagnostic groups. Median time to neutrophil engraftment was 11 days in non-CGD PIDs and 16 days in CGD patients. (C) Cumulative incidence of platelet engraftment (defined as >50 × 109/L for 3 consecutive days). Median time to platelet engraftment was 12 days in MRD transplants and 18 days in MUD/MMUD transplants. (D) Cumulative incidence of platelet engraftment in the 2 main diagnostic groups. Median time to platelet engraftment was 16 days in non-CGD PIDs and 19 days in CGD patients.
Median time to platelet engraftment in MRD transplants was 12 days (IQR, 11-21 days) and 18 days (IQR, 12-21 days) in MUD/MMUD transplants (Figure 2C). Longer platelet engraftment was also seen in CGD patients: median 19 days (IQR, 14-20 days) compared with 16 days (IQR, 11-21 days) in patients with other PIDs (Figure 2D). None of the observed differences were statistically significant. One patient had a CD34+ selected stem cell topup due to persistent cytopenias, with good effect.
Transplant-related mortality
Conditioning was generally well tolerated with no cases of interstitial pneumonitis, veno-occlusive disease, or severe mucositis (grade III-IV). TRM was low, with only 4 deaths observed at a median follow-up of 31 months for the whole cohort (n = 29) (Figure 3A-C). Two patients died during the neutropenic phase, prior to engraftment, of multiorgan failure secondary to sepsis (1 had no active infection immediately prior to transplant and the other had chronic cholangiopathy and was on antimicrobial prophylaxis), 1 at 7 months posttransplant of granulomatous meningoencephalitis, and a further patient at 28 months posttransplant secondary to sepsis in the context of chronic extensive GVHD.
Cumulative incidence of TRM. (A) TRM for all patients was 11% (n = 29) at 1 year and 15% at 3 years. (B) TRM for patients with MRDs was 10% at 1 year and 22% at 3 years (n = 11) vs 11% at 1 year and 3 years for MUD/MMUD transplants (n = 18; P = .536). (C) TRM for patients with CGD was 10% at 1 year and at 3 years (n = 11) vs other PID 12% at 1 year and 3 years (n = 18; P = .73).
Cumulative incidence of TRM. (A) TRM for all patients was 11% (n = 29) at 1 year and 15% at 3 years. (B) TRM for patients with MRDs was 10% at 1 year and 22% at 3 years (n = 11) vs 11% at 1 year and 3 years for MUD/MMUD transplants (n = 18; P = .536). (C) TRM for patients with CGD was 10% at 1 year and at 3 years (n = 11) vs other PID 12% at 1 year and 3 years (n = 18; P = .73).
Graft-versus-host disease
Thirteen patients developed grades I-II acute GVHD (10 skin only, 1 skin and gut, 1 gut only, and 1 liver only) and 1 patient developed grade III acute GVHD of the liver. Of these, 7 progressed to limited (single organ) chronic GVHD (4 skin only, 2 gut only. and 1 pulmonary). A further patient developed steroid-refractory extensive chronic GVHD. At last follow-up, no patients had ongoing active chronic GVHD requiring systemic immune suppression.
Infectious complications
A large proportion of patients had a high infectious burden pretransplant as expected in patients with PIDs. Of the 29 patients, 24 (82%) had prior recurrent or severe infections, including recurrent bacterial infection (n = 8), recurrent viral infection (n = 7), both bacterial and systemic fungal infections (n = 3), systemic fungal infection (n = 2), both viral and bacterial infection (n = 2), atypical mycobacterial infection (n = 1), and combined viral and atypical mycobacterial infection (n = 1). One patient had active pulmonary aspergilloma and 3 patients had ongoing human papilloma virus (HPV) infection at the time of transplant (indicated in Table 1). Despite this, there was a distinct absence of major infection issues both intra– and immediately post–Allo-HSCT in our cohort. No patients suffered serious fungal infection posttransplant, although azole prophylaxis was continued until off cyclosporin A (CSA) in patients with preexisting fungal infection (Table 2). No patients required granulocyte infusions.
Transplant outcome and resolution of PID-related complications
| . | Diagnosis . | Acute GVHD (grade) . | Chronic GVHD . | CMV status (R/D) . | Infectious complications . | Other complications . | Days F/U . | Present status . | Immuno- suppression at last F/U (Y/N) . | Ig replace-ment (continues, off, or N/A) . | Peripheral blood chimerism at last F/U (PBMC)* . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CVID | N/A | N/A | +/+ | Sepsis | Multi organ failure | 12 | Dead | Y | N/A | N/A | Died TRM (sepsis) |
| 2 | CVID | None | Probable pulmonary | +/+ | CMV reactivation. Recurrent bacterial chest infection | ITP. Progressive pulmonary fibrosis/bronchiolitis obliterans | 3434 | Dead | Y | Continued until death | Full donor chimerism | Died (progressive respiratory failure secondary to preexisting pulmonary fibrosis ± pulmonary GVHD) |
| 3 | APDS2 | Grade 1 gut | Limited (gut) | +/+ | Intermittent respiratory tract infections | Papillary renal cell carcinoma (unrelated) | 730 | Alive | N | Off | Full donor chimerism | Well |
| 4 | Autoimmune LPD | None | Limited post DLI | +/− | None | Esophageal stricture secondary to peptic ulceration. DLI ×3 for mixed chimerism | 4358 | Alive | N | N/A | Full donor chimerism | Well |
| 5 | Autoimmune LPD | None | None | −/− | None | CSA-induced neurotoxicity DLI ×4 for presumed PTLD (no biopsy, FDG-avid lymphadenopathy on CT/PET scan) | 3378 | Alive | N | Off | Full donor chimerism | Well. Neurotoxicity resolved |
| 6 | Cγ-chain SCID | N/A | N/A | +/+ | Sepsis | Multiorgan failure | 7 | Dead | Y | N/A | N/A | Died TRM (sepsis) |
| 7 | Absolute NK deficiency | Grade 1 skin | None | +/+ | Persistent planar warts (HPV2) | None | 2015 | Alive | N | Off | Full donor chimerism | Persistent extensive warts |
| 8 | DCML deficiency | Grade 2 skin and gut | None | +/+ | CMV reactivation (resolved). Radial excision for persistent HPV associated AIN and VIN | Acute thyroiditis (antibody negative). Ovarian failure | 1221 | Alive | N | N/A | Full donor chimerism | Well. Thyroiditis resolved. GVHD resolved |
| 9 | AR IL12Recβ deficiency | Grade 1 skin | Limited (gut) | −/− | EBV reactivation treated with rituximab | None | 1083 | Alive | N | Off | Full donor chimerism | Well |
| 10 | Rag2/red cell aplasia | None | None | −/− | None | Delayed engraftment | 700 | Alive | N | Off | Stable mixed chimerism (full donor in B-cell fraction, mixed in other lineages) | Well. Resolution of granulomatous skin lesions on shins |
| 11 | X-linked LPD | None | None | +/+ | BK virus cystitis | None | 3692 | Alive | N | Off | Stable mixed chimerism (mixed all lineages) | Well. Normal spermatozoa |
| 12 | Combined immune deficiency | Grade 2 skin | None | +/+ | CMV reactivation | Intermittent neutropenia | 1042 | Alive | N | Continues | Stable mixed chimerism (mixed all lineages) | Well |
| 13 | CD27 deficiency | Grade 2 skin | Limited (skin) | −/− | Rhinovirus. EBV reactivation treated with rituximab | None | 544 | Alive | N | Continues | Full donor chimerism | Well. CT/PET ongoing remission |
| 14 | Gata2 deficiency | None | None | +/− | Persistent perineal HPV with VIN3 | DLI ×3 for MC. Fibromyalgia. Chronic fatigue. Thyrotoxicosis | 1051 | Alive | N | N/A | Full donor chimerism | Well. Resolution of warts on hands and feet |
| 15 | XIAP deficiency | None | None | −/+ | Warts left foot and right index finger resolving | None | 629 | Alive | N | N/A | Stable mixed chimerism (mixed all lineages) | Well. No further colitis |
| 16 | Autoimmune LPD | Grade 2 skin | None | +/+ | CMV reactivation ×1 | Biopsy proven EBV PTLD treated with 4 cycles rituximab | 118 | Alive | N | N/A | Chimerism pending | Well. No further CMV or EBV reactivations |
| 17 | Gata 2 mutation | Grade 1 skin | None | −/− | Low-level EBV reactivation not requiring intervention | None | 209 | Alive | Y | N/A | Full donor chimerism | Well. Weaning cyclosporin |
| 18 | XIAP deficiency | None | None | +/− | None | Slow recovery of counts and persistent splenomegaly | 115 | Alive | Y | Off | Full donor chimerism | Well. Weaning cyclosporin. Trial off Ig as IgG in normal range at 4 mo post |
| 19 | AR-CGD | None | None | +/+ | CMV reactivation | Iron and vitamin D deficiency | 983 | Alive | N | N/A | Stable mixed chimerism (mixed all lineages) | Well. Viable sperm |
| 20 | AR-CGD | None | None | −/− | None | None | 971 | Alive | N | N/A | Stable mixed chimerism (full donor in PBMCs and B cells, mixed in T-cell and granulocyte fractions) | Well |
| 21 | AR-CGD | None | None | +/+ | Bilateral lower lobe consolidation (no organisms identified) | Hemophagocytosis and prolonged cytopenias; granulomatous meningitis (no pathogen identified); acute hepatic failure | 210 | Dead | Y | N/A | Full donor chimerism | Died TRM (multiorgan failure) |
| 22 | Variant CGD | Grade 1 skin | None | −/− | None | Premature ovarian insufficiency | 992 | Alive | N | N/A | Stable mixed chimerism (full donor in B-cell fraction, mixed in other lineages) | Well. Resolution of granulomatous colitis |
| 23 | X-linked CGD | Grade 3 liver | Steroid refractory, extensive | +/− | Multiple infective complications associated with immune suppression for GVHD | 616 | Dead | Y | N/A | Full donor chimerism | Died TRM (steroid refractory extensive chronic GVHD) | |
| 24 | X-linked CGD | None | None | −/− | None | Mild renal impairment | 1858 | Alive | N | N/A | Stable mixed chimerism (mixed in all lineages) | Well. Renal impairment resolved |
| 25 | X-linked CGD | Grade 1 skin | None | −/− | Adenoviraemia | EBV PTLD treated with 4 cycles rituximab | 1003 | Alive | N | N/A | Full donor chimerism | Well |
| 26 | X-linked CGD | Grade 1 skin | Limited (skin) | −/− | Pulmonary aspergillosis | Renal impairment (resolved), CD34+ topup for prolonged cytopenias | 1319 | Alive | N | N/A | Full donor chimerism | Well. Some viable sperm suitable for ICSI |
| 27 | X-linked CGD | Grade 1 skin | Limited (skin) | −/− | None | None | 857 | Alive | N | N/A | Stable mixed chimerism (full donor in PBMC and B-cell fractions, mixed in T-cell and granulocyte fractions) | Well. Viable sperm on testing, suitable for ICSI |
| 28 | X-linked CGD | None | None | +/− | None | DLI ×4 for mixed chimerism | 3516 | Alive | N | N/A | Stable mixed chimerism (mixed in all lineages) | Well. Normal full blood count. Father of 2 children |
| 29 | X-linked CGD | None | None | −/− | Rotavirus diarrhea | Depression | 697 | Alive | N | N/A | Stable mixed chimerism (full donor in PBMC and B-cell fractions, mixed in T-cell and granulocyte fractions) | Well |
| . | Diagnosis . | Acute GVHD (grade) . | Chronic GVHD . | CMV status (R/D) . | Infectious complications . | Other complications . | Days F/U . | Present status . | Immuno- suppression at last F/U (Y/N) . | Ig replace-ment (continues, off, or N/A) . | Peripheral blood chimerism at last F/U (PBMC)* . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CVID | N/A | N/A | +/+ | Sepsis | Multi organ failure | 12 | Dead | Y | N/A | N/A | Died TRM (sepsis) |
| 2 | CVID | None | Probable pulmonary | +/+ | CMV reactivation. Recurrent bacterial chest infection | ITP. Progressive pulmonary fibrosis/bronchiolitis obliterans | 3434 | Dead | Y | Continued until death | Full donor chimerism | Died (progressive respiratory failure secondary to preexisting pulmonary fibrosis ± pulmonary GVHD) |
| 3 | APDS2 | Grade 1 gut | Limited (gut) | +/+ | Intermittent respiratory tract infections | Papillary renal cell carcinoma (unrelated) | 730 | Alive | N | Off | Full donor chimerism | Well |
| 4 | Autoimmune LPD | None | Limited post DLI | +/− | None | Esophageal stricture secondary to peptic ulceration. DLI ×3 for mixed chimerism | 4358 | Alive | N | N/A | Full donor chimerism | Well |
| 5 | Autoimmune LPD | None | None | −/− | None | CSA-induced neurotoxicity DLI ×4 for presumed PTLD (no biopsy, FDG-avid lymphadenopathy on CT/PET scan) | 3378 | Alive | N | Off | Full donor chimerism | Well. Neurotoxicity resolved |
| 6 | Cγ-chain SCID | N/A | N/A | +/+ | Sepsis | Multiorgan failure | 7 | Dead | Y | N/A | N/A | Died TRM (sepsis) |
| 7 | Absolute NK deficiency | Grade 1 skin | None | +/+ | Persistent planar warts (HPV2) | None | 2015 | Alive | N | Off | Full donor chimerism | Persistent extensive warts |
| 8 | DCML deficiency | Grade 2 skin and gut | None | +/+ | CMV reactivation (resolved). Radial excision for persistent HPV associated AIN and VIN | Acute thyroiditis (antibody negative). Ovarian failure | 1221 | Alive | N | N/A | Full donor chimerism | Well. Thyroiditis resolved. GVHD resolved |
| 9 | AR IL12Recβ deficiency | Grade 1 skin | Limited (gut) | −/− | EBV reactivation treated with rituximab | None | 1083 | Alive | N | Off | Full donor chimerism | Well |
| 10 | Rag2/red cell aplasia | None | None | −/− | None | Delayed engraftment | 700 | Alive | N | Off | Stable mixed chimerism (full donor in B-cell fraction, mixed in other lineages) | Well. Resolution of granulomatous skin lesions on shins |
| 11 | X-linked LPD | None | None | +/+ | BK virus cystitis | None | 3692 | Alive | N | Off | Stable mixed chimerism (mixed all lineages) | Well. Normal spermatozoa |
| 12 | Combined immune deficiency | Grade 2 skin | None | +/+ | CMV reactivation | Intermittent neutropenia | 1042 | Alive | N | Continues | Stable mixed chimerism (mixed all lineages) | Well |
| 13 | CD27 deficiency | Grade 2 skin | Limited (skin) | −/− | Rhinovirus. EBV reactivation treated with rituximab | None | 544 | Alive | N | Continues | Full donor chimerism | Well. CT/PET ongoing remission |
| 14 | Gata2 deficiency | None | None | +/− | Persistent perineal HPV with VIN3 | DLI ×3 for MC. Fibromyalgia. Chronic fatigue. Thyrotoxicosis | 1051 | Alive | N | N/A | Full donor chimerism | Well. Resolution of warts on hands and feet |
| 15 | XIAP deficiency | None | None | −/+ | Warts left foot and right index finger resolving | None | 629 | Alive | N | N/A | Stable mixed chimerism (mixed all lineages) | Well. No further colitis |
| 16 | Autoimmune LPD | Grade 2 skin | None | +/+ | CMV reactivation ×1 | Biopsy proven EBV PTLD treated with 4 cycles rituximab | 118 | Alive | N | N/A | Chimerism pending | Well. No further CMV or EBV reactivations |
| 17 | Gata 2 mutation | Grade 1 skin | None | −/− | Low-level EBV reactivation not requiring intervention | None | 209 | Alive | Y | N/A | Full donor chimerism | Well. Weaning cyclosporin |
| 18 | XIAP deficiency | None | None | +/− | None | Slow recovery of counts and persistent splenomegaly | 115 | Alive | Y | Off | Full donor chimerism | Well. Weaning cyclosporin. Trial off Ig as IgG in normal range at 4 mo post |
| 19 | AR-CGD | None | None | +/+ | CMV reactivation | Iron and vitamin D deficiency | 983 | Alive | N | N/A | Stable mixed chimerism (mixed all lineages) | Well. Viable sperm |
| 20 | AR-CGD | None | None | −/− | None | None | 971 | Alive | N | N/A | Stable mixed chimerism (full donor in PBMCs and B cells, mixed in T-cell and granulocyte fractions) | Well |
| 21 | AR-CGD | None | None | +/+ | Bilateral lower lobe consolidation (no organisms identified) | Hemophagocytosis and prolonged cytopenias; granulomatous meningitis (no pathogen identified); acute hepatic failure | 210 | Dead | Y | N/A | Full donor chimerism | Died TRM (multiorgan failure) |
| 22 | Variant CGD | Grade 1 skin | None | −/− | None | Premature ovarian insufficiency | 992 | Alive | N | N/A | Stable mixed chimerism (full donor in B-cell fraction, mixed in other lineages) | Well. Resolution of granulomatous colitis |
| 23 | X-linked CGD | Grade 3 liver | Steroid refractory, extensive | +/− | Multiple infective complications associated with immune suppression for GVHD | 616 | Dead | Y | N/A | Full donor chimerism | Died TRM (steroid refractory extensive chronic GVHD) | |
| 24 | X-linked CGD | None | None | −/− | None | Mild renal impairment | 1858 | Alive | N | N/A | Stable mixed chimerism (mixed in all lineages) | Well. Renal impairment resolved |
| 25 | X-linked CGD | Grade 1 skin | None | −/− | Adenoviraemia | EBV PTLD treated with 4 cycles rituximab | 1003 | Alive | N | N/A | Full donor chimerism | Well |
| 26 | X-linked CGD | Grade 1 skin | Limited (skin) | −/− | Pulmonary aspergillosis | Renal impairment (resolved), CD34+ topup for prolonged cytopenias | 1319 | Alive | N | N/A | Full donor chimerism | Well. Some viable sperm suitable for ICSI |
| 27 | X-linked CGD | Grade 1 skin | Limited (skin) | −/− | None | None | 857 | Alive | N | N/A | Stable mixed chimerism (full donor in PBMC and B-cell fractions, mixed in T-cell and granulocyte fractions) | Well. Viable sperm on testing, suitable for ICSI |
| 28 | X-linked CGD | None | None | +/− | None | DLI ×4 for mixed chimerism | 3516 | Alive | N | N/A | Stable mixed chimerism (mixed in all lineages) | Well. Normal full blood count. Father of 2 children |
| 29 | X-linked CGD | None | None | −/− | Rotavirus diarrhea | Depression | 697 | Alive | N | N/A | Stable mixed chimerism (full donor in PBMC and B-cell fractions, mixed in T-cell and granulocyte fractions) | Well |
Full donor indicates ≥97% donor DNA; mixed chimerism indicates ≥50% <97% donor DNA.
BK, BK virus; CT, computed tomography; DLI, donor lymphocyte infusion; EBV, Epstein-Barr virus; FDG, fluorodeoxyglucose; F/U, follow-up; GVHD, graft-versus-host disease; ICSI, intracytoplasmic sperm injection; ITP, idiopathic thrombocytopenic purpura; MC, mixed chimerism; N, no; N/A, not applicable; PET, positron emission tomography; PTLD, posttransplant lymphoproliferative disease; TRM, transplant-related mortality; VIN, vaginal intraepithelial neoplasia; Y, yes. Other abbreviations are explained in Table 1.
See Figure 4 for lineage-specific chimerism.
CMV reactivation was only observed in 6 patients (35% of at-risk patients, defined as +/+, −/+, or +/− recipient/donor pairs) and in all cases these responded to standard antiviral therapy. EBV reactivation with evidence of posttransplant lymphoproliferative disease (PTLD) was observed in 4 patients, all of whom responded completely to rituximab. An additional patient was treated with donor lymphocyte infusions (DLIs) for presumed PTLD where no biopsy was available (n = 1). No patient died of CMV, EBV, or adenovirus infection.
Five patients had persistent viral warts pretransplant with complete resolution or ongoing resolution of warts observed at last follow-up in 2 patients. In 3 patients, no improvement in warts was observed, despite full donor T-cell chimerism in all, including the 2 patients transplanted for dendritic cell, monocyte, B lymphocyte, and natural killer (NK) lymphocyte (DCML) deficiency, who had preexisting extensive perineal HPV-related intraepithelial neoplasia (vaginal intraepithelial neoplasia [VIN], cervical intraepithelial neoplasia, or anal intraepithelial neoplasia [AIN]). A further patient with extensive confluent warts pretransplant (NK deficiency) had persistent warts despite full reconstitution of T, B, and NK cells.
At last follow-up, the remaining 21 patients had no evidence of persistent or recurrent infections.
Immune reconstitution
Lymphocyte subset analysis was performed on all of the PID patients pretransplant. Lymphocyte subset analysis was performed on all patients surviving beyond 3 months posttransplant (n = 27) within the first 12 months posttransplant. Of the non-CGD PID patients (n = 18), 13 of the 16 surviving PID patients had lymphocyte subset results at 12 months (3 had not reached 12 months follow-up at the time of data collection). Seventy percent of those with subset analysis performed (n = 13) had achieved a normal lymphocyte count (1.0-2.8 × 109/L), 62% had normal absolute CD3+ cell counts (0.7-2.1 × 109/L), 62% normal CD4+ cell counts (0.3-1.4 × 109/L) and 77% normal CD8+ cell counts (0.2-0.9 × 109/L).
All CGD patients had normal neutrophil function tests posttransplant (data not shown).
Of the 9 surviving patients who had been receiving monthly immunoglobulin replacement therapy pretransplant, 89% were immunoglobulin-free at last follow-up (Table 2).
Chimerism
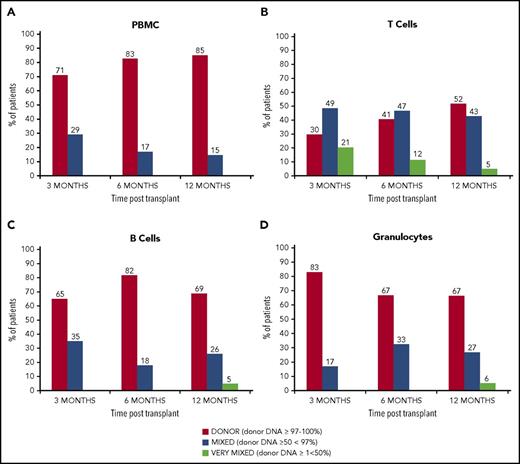
Chimerism data were available for 23 of the 24 surviving patients at last follow-up. At the time of analysis, 21 patients had results available at 12 months posttransplant and 85% had full donor chimerism in unfractionated PBMCs. In the T-cell fraction, 52% of patients tested had achieved full donor chimerism at 12 months (Figure 4).
Chimerism kinetics. Peripheral blood chimerism posttransplant. (A) PBMC chimerism results at 3, 6, and 12 months (n = 20, 17, and 19, respectively): full donor chimerism (≥97% donor DNA) was achieved in PBMC fraction in 71% at 3 months, 83% at 6 months, and 85% at 12 months. (B) T-cell chimerism results: full donor chimerism was achieved in the T-cell fraction in 30% at 3 months, 41% at 6 months, and 52% at 12 months. (C) B-cell chimerism: full donor chimerism was achieved in the B-cell fraction in 65% at 3 months, 82% at 6 months, and 69% at 12 months. (D) Chimerism in the granulocyte fraction. Full donor chimerism was seen in 83% patients at 3 months, 67% at 6 months, and 67% at 12 months.
Chimerism kinetics. Peripheral blood chimerism posttransplant. (A) PBMC chimerism results at 3, 6, and 12 months (n = 20, 17, and 19, respectively): full donor chimerism (≥97% donor DNA) was achieved in PBMC fraction in 71% at 3 months, 83% at 6 months, and 85% at 12 months. (B) T-cell chimerism results: full donor chimerism was achieved in the T-cell fraction in 30% at 3 months, 41% at 6 months, and 52% at 12 months. (C) B-cell chimerism: full donor chimerism was achieved in the B-cell fraction in 65% at 3 months, 82% at 6 months, and 69% at 12 months. (D) Chimerism in the granulocyte fraction. Full donor chimerism was seen in 83% patients at 3 months, 67% at 6 months, and 67% at 12 months.
Multilineage full donor chimerism was observed in 10 of the patients (48%) with the rest showing mixed chimerism in at least 1 of the cell lineages tested. A trend to increasing chimerism stability with time was observed in our cohort. Chimerism was more robust in the B and myeloid cells compared with the T-cell fraction. No correlation was found between mixed donor chimerism and age of patient, underlying diagnosis or donor type.
Two patients received DLI for persistent mixed chimerism (1 in the context of presumed PTLD with rising B-cell numbers and FDG-avid lymphadenopathy on PET/CT scan). One achieved full donor chimerism in B cells and granulocytes with T cells remaining mixed and the other converted to full donor chimerism.
There were no cases of graft rejection, and, in all surviving patients, either stable mixed chimerism or full donor chimerism were observed.
PID-associated colitis
Of the 10 patients with moderate-severe inflammatory bowel disease pretransplant (6 CGD, 1 XIAP, 1 CVID, 1 autoimmune lymphoproliferative syndrome and 1 DCML deficiency), 2 died of TRM (both with CGD, patients 21 and 23), and the colitis has resolved in all 8 surviving patients, including the XIAP patient. No increased incidence of gut GVHD was observed in these patients.
Discussion
We have demonstrated that in 29 young adult patients with high-risk primary immune deficiencies, reduced-intensity, in vivo T-cell–depleted Allo-HSCT was both effective and safe, with an OS of 85.2% at 3 years and a mean follow-up of 3.5 years (41 months). There was no significant difference in outcome between those undergoing MRD transplants and matched, or 1 antigen MMUD transplants (P = .51). As predicted, using our previously described T-cell–depleting conditioning regimens, the observed cumulative incidence of severe acute GVHD incidence (grades III-IV) was very low at 6.5% and only 31% of patients developed chronic GVHD, symptoms which resolved allowing withdrawal of systemic immune suppression from 3 months posttransplant in all but 1 of these patients. Full multilineage donor chimerism was achieved in 42% of patients, and all others achieved stable mixed chimerism. Larger studies are required to determine the degree of donor chimerism required posttransplant for some of the rarer PIDs in order to achieve a functional cure. Good functional immune reconstitution was observed in all but 1 of the patients, permitting the withdrawal of immunoglobulin replacement therapy posttransplant in 89% of patients. At last follow-up, 92% of surviving patients (n = 24) were off of immune suppression and the remaining 2 were in the process of weaning.
The OS and EFS observed in this series of adult patients is comparable or better than that seen in published series of Allo-HSCT outcomes for pediatric and adolescent patients with PID and CGD.9,23,34-36
The patients in this study had not undergone Allo-HSCT earlier in life due to a variety of reasons including mild-moderate clinical phenotype in childhood therefore not precipitating referral, delay in diagnosis until adolescence/adulthood, late presentation, and/or lack of a suitable donor. Patients had subsequently developed complications that necessitated definitive treatment in the form of Allo-HSCT. Triggers for referral included life-threatening infection, malignancy, autoimmune or inflammatory phenomena, newly confirmed genetic diagnosis or new donor availability. Due to preexisting organ dysfunction, ongoing infectious or inflammatory phenomena, all patients in this cohort had a HCT-CI of at least 1 whereas 12 had a score of 3 or greater predicting a higher than observed TRM.25,37 Both the HCT-CI score or European Group for Blood and Bone Marrow Transplantation (EBMT) score have been validated in patients with hematological malignancies and in pediatric populations.38,39 Neither score has been validated in patients with PID or specifically CGD, however, it provides validated information on the clinical condition of patients pretransplant. Patients with cellular immunodeficiency have been shown to have a higher HCT-CI and this score is used most frequently in other published studies of HSCT in PIDs.25
These promising results suggest that Allo-HSCT is safe when delivered in a specialist center and should be considered as a potentially curative option for younger adult PID patients with an appropriate donor and a sufficiently severe clinical picture. We recommend proceeding to Allo-HSCT for adult patients with a known genetic diagnosis amenable to correction by transplantation, and where conservative management results in a shortened life expectancy and ongoing morbidity. The current widespread use of next-generation sequencing is expected to facilitate earlier referral of eligible adults.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stuart Ings and Fiona O’Brien for their assistance in collecting data on CD34 cell doses.
E.M., K.T., and S.B. are supported by the University College London (UCL) Hospital (UCLH) National Institute of Health Research (NIHR) Biomedical Research Centre. A number of coauthors receive funding from the NIHR (E.M., S.B., and K.P.), the NIHR UCLH/UCL Biomedical Research Centre (E.M., S.B., D.L., and K.T.), the NIHR GOSH/UCL Biomedical Research Centre (M.B.), Bloodwise (E.M., R.C., A.F., and K.P.), Wellcome Trust (R.C. and V.B.), Medical Research Council (E.M. and R.C.), Cancer Research UK (CRUK) (R.C., A.F., and K.P.), The CRUK Experimental Cancer Medicine Centre (E.M. and K.P.), Teenage Cancer Trust (TYA BMT unit), and the UK Primary Immunodeficiency Network (M.B. and D.L.).
Authorship
Contribution: T.A.F., S.B., K.T., S.G., and E.M. collected the data and wrote the manuscript; all other authors provided clinical care for the patients described; all authors had access to the clinical and laboratory data; and E.M. had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emma Morris, UCL Institute of Immunity & Transplantation, 2nd Floor, Royal Free London Hospital, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: e.morris@ucl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal