Key Points
VWF sialylation modulates in vivo clearance through Ashwell-Morrell independent pathways.
VWF binding to MGL plays a novel role in facilitating VWF clearance.
Abstract
Previous studies have shown that loss of terminal sialic acid causes enhanced von Willebrand factor (VWF) clearance through the Ashwell-Morrell receptor (AMR). In this study, we investigated (1) the specific importance of N- vs O-linked sialic acid in protecting against VWF clearance and (2) whether additional receptors contribute to the reduced half-life of hyposialylated VWF. α2-3-linked sialic acid accounts for <20% of total sialic acid and is predominantly expressed on VWF O-glycans. Nevertheless, specific digestion with α2-3 neuraminidase (α2-3Neu-VWF) was sufficient to cause markedly enhanced VWF clearance. Interestingly, in vivo clearance experiments in dual VWF−/−/Asgr1−/− mice demonstrated enhanced clearance of α2-3Neu-VWF even in the absence of the AMR. The macrophage galactose-type lectin (MGL) is a C-type lectin that binds to glycoproteins expressing terminal N-acetylgalactosamine or galactose residues. Importantly, the markedly enhanced clearance of hyposialylated VWF in VWF−/−/Asgr1−/− mice was significantly attenuated in the presence of an anti-MGL inhibitory antibody. Furthermore, dose-dependent binding of human VWF to purified recombinant human MGL was confirmed using surface plasmon resonance. Additionally, plasma VWF:Ag levels were significantly elevated in MGL1−/− mice compared with controls. Collectively, these findings identify MGL as a novel macrophage receptor for VWF that significantly contributes to the clearance of both wild-type and hyposialylated VWF.
Introduction
Although substantial progress has been achieved in understanding von Willebrand factor (VWF) structure and function, the biological mechanisms underpinning VWF clearance from the plasma remain poorly understood.1 Nevertheless, studies have demonstrated that enhanced VWF clearance plays an important role in the etiology of both type 1 and type 2 von Willebrand disease (VWD).1,2 During biosynthesis, VWF undergoes complex posttranslational modification, including significant N- and O-linked glycosylation. Mass spectrometry studies have shown that sialylated biantennary complex-type chains constitute the commonest N-linked glycans expressed on VWF, whereas a disialylated core 1 tetrasaccharide structure (known as the T antigen) accounts for 70% of the total O-glycan population.3,4 Thus, the majority of N- and O-linked glycans of human VWF are capped by negatively charged sialic acid residues.5 In keeping with other plasma glycoproteins, terminal sialic acid expression plays an important role in protecting VWF against clearance.6,7 Consequently, enzymatic removal of terminal sialylation from VWF has been associated with a markedly reduced plasma half-life in vivo.8 Similarly, genetic inactivation of ST3GalIV sialyltransferase in a transgenic mouse model causes enhanced VWF clearance.9 Several studies have reported significantly reduced VWF sialylation levels in patients with type 1 VWD.7,9 Furthermore, van Schooten et al reported an inverse correlation between aberrant sialylation of T antigen and plasma VWF:Ag levels, suggesting that O-linked sialylation on VWF may be of particular importance.7
Current evidence suggests that the enhanced clearance of hyposialylated VWF occurs via the Ashwell-Morrell receptor (AMR).10 This C-type lectin is expressed on hepatocytes and is composed of 2 transmembrane protein subunits (Asgpr-1 and Asgpr-2). Grewal et al previously demonstrated that plasma VWF clearance is significantly attenuated in Asgr-1 knockout mice.10 Nevertheless, important questions regarding the biological mechanisms through which VWF sialylation regulates its clearance in vivo remain unclear. In particular, the relative importance of N-linked vs O-linked sialylation in regulating physiological and/or pathological clearance of VWF has not been defined. In addition to the AMR, a number of other lectin receptors have been shown to bind with enhanced affinity to hyposialylated glycoproteins.11 In this study, we demonstrate a critical role for VWF O-linked sialylation in modulating in vivo clearance and further define a novel role for the macrophage galactose-type lectin (MGL) in regulating VWF clearance in a sialic acid–dependent manner.
Study design
Isolation and digestion of human plasma–derived VWF
As described in the supplemental Methods (available on the Blood Web site), plasma-derived VWF (pd-VWF) was purified from commercial concentrate Fandhi (Grifols, Barcelona, Spain) and subsequently treated with α2-3 neuraminidase (Streptococcus pneumonia; Sigma Aldrich, Ireland) or α2-3,6,8,9 neuraminidase (Arthrobacter ureafaciens; New England Biolabs, United Kingdom) as previously described.5 VWF glycan expression was analyzed using lectin enzyme-linked immunosorbent assays (see supplemental Methods and supplemental Figure 1).8
VWF clearance studies
VWF−/− and Asgr1−/− mice on a C57BL/6J background were obtained from the Jackson Laboratory (Sacramento, CA) and crossbred to generate novel VWF−/−/Asgr1−/− double-knockout mice. MGL1−/− mice were also obtained from the Jackson Laboratory. Where indicated, clearance studies were repeated in the presence of either clodronate or asialo-orosomucoid (ASOR) as previously described.8,12 Specific clearance studies were performed after inhibition of MGL using a polyclonal goat anti-mouse MGL1/2 antibody. All in vivo clearance experiments were performed as detailed in the supplemental Methods in accordance with the Health Product Regulatory Authority, Ireland.
In vitro VWF binding studies
As described in the supplemental Methods, surface plasmon resonance (SPR) was used to evaluate MGL binding to VWF.13 Briefly, purified pd-VWF was immobilized on a CM5 chip, and binding to recombinant MGL (R&D Systems, United Kingdom) was determined. Furthermore, proximity ligation assay (Duolink-PLA; Sigma Aldrich, Ireland) was performed to evaluate colocalization of VWF and MGL on THP1 macrophages.
Data presentation and statistical analysis
Experimental data were analyzed with GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Data were expressed as mean values ± standard error of the mean (SEM). Data were analyzed with Student unpaired 2-tailed t test, and P values of <.05 were deemed significant.
Results and discussion
VWF sialylation modulates clearance through Ashwell-Morrell independent pathways
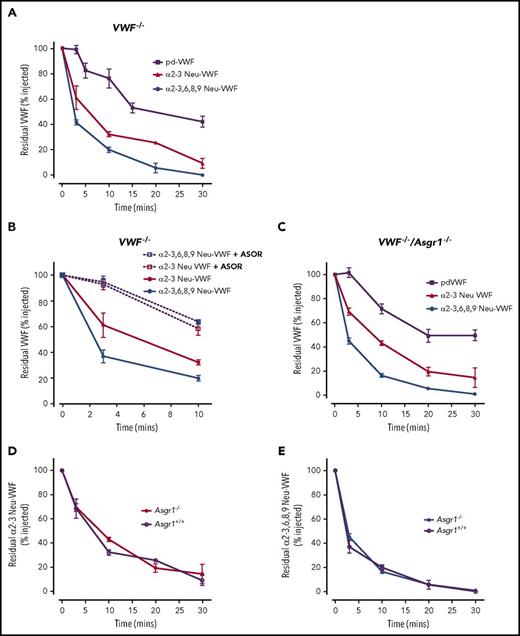
In keeping with previous studies, we observed that combined removal of N- and O-linked sialic acid by digestion with α2-3,6,8,9 neuraminidase resulted in markedly enhanced clearance of pd-VWF in VWF−/− mice (Figure 1A). Specific removal of α2-3-linked sialic acid was sufficient to markedly enhance VWF clearance (t1/2 = 9.0 ± 1 minutes; P < .05) (Figure 1A). In fact, clearance of α2-3 Neu-VWF was almost as rapid as that of α2-3,6,8,9 Neu-VWF (t1/2 = 4.0 ± 0.3 minutes). This finding is interesting because α2-3-linked sialic acid is predominantly located on the O-linked glycans of human VWF and accounts for <20% of total sialic acid expression.5 Currently, the AMR is the only receptor described to regulate clearance of hyposialylated VWF.10 We found that the enhanced clearance of α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF were both significantly attenuated in the presence of a hyposialylated inhibitor glycoprotein (ASOR) (Figure 1B). However, ASOR has a short plasma half-life and is not a specific AMR inhibitor.14 Previous studies have shown that AMR demonstrates significantly greater affinity for exposed galactose residues on tri- and tetra-antennary galactoses (as present on VWF N-glycan) compared with terminal galactose moieties on mono- or biantennary galactoses (as present on VWF O-glycan).15,16 We therefore hypothesized that other lectin receptors may contribute to the enhanced clearance of hyposialylated VW and be of particular importance in modulating the effects of O-linked sialylation on VWF clearance. To address this, in vivo clearance studies were repeated in dual VWF−/−/Asgr1−/− knockout mice. Critically, we observed that markedly enhanced clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF persisted in VWF−/−/Asgr1−/− mice (t1/2 = 8.2 ± 0.6 and 3.2 ± 0.4 vs 50.6 ± 2 minutes for pd-VWF; P < .05) (Figure 1C). Furthermore, the enhanced clearance rates observed for α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF were not significantly different in the presence or absence of the AMR (Figure 1D-E). Collectively, these data confirm that reductions in N- and/or O-linked sialylation have major effects on VWF half-life and demonstrate that α2-3-linked sialic acid expressed on O-linked glycans may be of particular importance in regulating pd-VWF clearance. Furthermore, our findings suggest that previously unrecognized AMR-independent pathways contribute to the enhanced clearance of hyposialylated VWF in vivo.
Clearance of hyposialylated VWF proceeds independently of AMR. (A) To study the effects of N- and O-linked sialylation on VWF clearance, purified human pd-VWF was treated with either α2-3,6,8,9 or α2-3 neuraminidase. In vivo clearance for each glycoform was then assessed in VWF−/− mice and compared with that of wild-type pd-VWF. At each time point, residual circulating VWF concentration was determined by VWF:Ag enzyme-linked immunosorbent assay. All results are plotted as percentage residual VWF:Ag levels relative to the amount injected. Three to 5 mice were used per time point. Data are represented as mean ± SEM. In some cases, the SEM cannot be seen because of its small size. (B) In the presence of ASOR, the enhanced in vivo clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF was significantly attenuated (α2-3 Neu-VWF t1/2 = 8.2 ± 1.4 minutes vs 12.4 ± 2.4 minutes, P < .05; and α2-3,6,8,9 Neu-VWF t1/2 = 3.7 ± 0.7 minutes vs 14.4 ± 2.7 minutes, P < .005, respectively). (C) To determine whether AMR-independent pathways contribute to the enhance clearance of hyposialylated VWF, in vivo clearance studies were repeated in VWF−/−/Asgr1−/− mice. Importantly, the markedly enhanced clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF was still evident in the absence of the AMR (t1/2 = 8.2 ± 0.6 and 3.2 ± 0.4 compared with 50.6 ± 2 minutes for pd-VWF; P < .05). Furthermore, the reduced half-life observed for α2-3 Neu-VWF (D) and α2-3,6,8,9 Neu-VWF (E) were not significantly different in the presence or absence of the AMR (α2-3 Neu-VWF t1/2 = 8.2 ± 1.4 minutes vs 8.2 ± 0.6 minutes, P = .96; and α2-3,6,8,9 Neu-VWF t1/2 = 3.7 ± 0.7 minutes vs 3.2 ± 0.4 minutes, P = .42, respectively).
Clearance of hyposialylated VWF proceeds independently of AMR. (A) To study the effects of N- and O-linked sialylation on VWF clearance, purified human pd-VWF was treated with either α2-3,6,8,9 or α2-3 neuraminidase. In vivo clearance for each glycoform was then assessed in VWF−/− mice and compared with that of wild-type pd-VWF. At each time point, residual circulating VWF concentration was determined by VWF:Ag enzyme-linked immunosorbent assay. All results are plotted as percentage residual VWF:Ag levels relative to the amount injected. Three to 5 mice were used per time point. Data are represented as mean ± SEM. In some cases, the SEM cannot be seen because of its small size. (B) In the presence of ASOR, the enhanced in vivo clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF was significantly attenuated (α2-3 Neu-VWF t1/2 = 8.2 ± 1.4 minutes vs 12.4 ± 2.4 minutes, P < .05; and α2-3,6,8,9 Neu-VWF t1/2 = 3.7 ± 0.7 minutes vs 14.4 ± 2.7 minutes, P < .005, respectively). (C) To determine whether AMR-independent pathways contribute to the enhance clearance of hyposialylated VWF, in vivo clearance studies were repeated in VWF−/−/Asgr1−/− mice. Importantly, the markedly enhanced clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF was still evident in the absence of the AMR (t1/2 = 8.2 ± 0.6 and 3.2 ± 0.4 compared with 50.6 ± 2 minutes for pd-VWF; P < .05). Furthermore, the reduced half-life observed for α2-3 Neu-VWF (D) and α2-3,6,8,9 Neu-VWF (E) were not significantly different in the presence or absence of the AMR (α2-3 Neu-VWF t1/2 = 8.2 ± 1.4 minutes vs 8.2 ± 0.6 minutes, P = .96; and α2-3,6,8,9 Neu-VWF t1/2 = 3.7 ± 0.7 minutes vs 3.2 ± 0.4 minutes, P = .42, respectively).
The macrophage galactose receptor regulates in vivo clearance of VWF
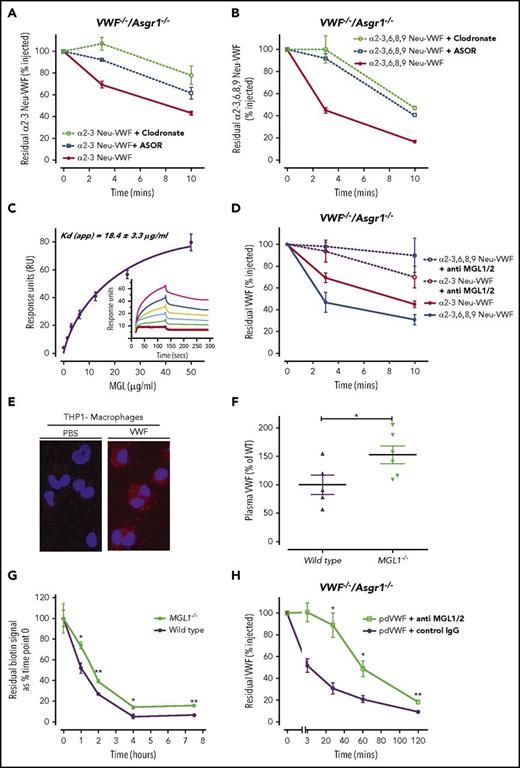
To investigate other receptors and/or cell types that modulate the enhanced clearance of hyposialylated VWF, α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF clearance studies in VWF−/−/Asgr1−/− mice were repeated in the presence of ASOR, or following clodronate-induced macrophage depletion (Figure 2A-B). The enhanced clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF was still inhibited by ASOR even in the absence of the AMR. Interestingly, in vivo macrophage depletion also significantly attenuated the enhanced clearance of hyposialylated VWF. Finally, in vitro binding studies demonstrated enhanced binding of asialo-VWF to differentiated THP1 macrophages (supplemental Figure 2). Collectively, these data demonstrate that additional asialo-receptors, at least in part expressed on macrophages, regulate the enhanced clearance of hyposialylated VWF in vivo.
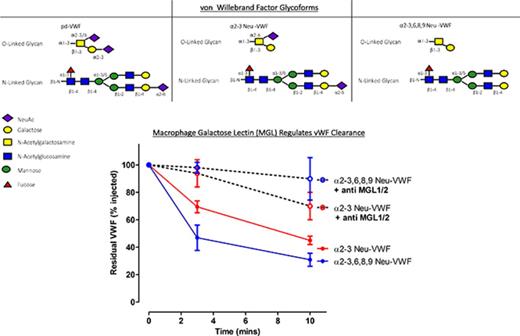
MGL facilitates VWF clearance in vivo. To investigate other receptors and/or cell types that modulate the enhanced clearance of hyposialylated VWF, α2-3 Neu-VWF (A) and α2-3,6,8,9 Neu-VWF (B) clearance studies in VWF−/−Asgr1−/− mice were repeated in the presence of ASOR, or following clodronate-induced macrophage depletion. The enhanced clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF was still inhibited by ASOR (blue lines) even in the absence of the AMR (α2-3 Neu-VWF t1/2 = 8.2 ± 1.4 minutes vs 16.8 ± 1.6 minutes, P < .005; and α2-3,6,8,9 Neu-VWF t1/2 = 3.7 ± 0.7 minutes vs 7.7 ± 1.3 minutes, P < .05, respectively). In addition, clodronate-induced macrophage depletion (green lines) also significantly attenuated the enhanced clearance of hyposialylated VWF (α2-3 Neu-VWF t1/2 = 8.2 ± 1.4 minutes vs 24.0 ± 1.1 minutes, P < .05; and α2-3,6,8,9 Neu-VWF t1/2 = 3.7 ± 0.7 minutes vs 9.6 ± 4.1 minutes, P < .05, respectively). Three to 5 mice were used per time point, and data are represented as mean ± SEM. (C) SPR was used to evaluate the binding of immobilized purified pd-VWF to recombinant human MGL. Dose-dependent binding were observed, with Kd (app) of 18.4 ± 3 µg/mL. (D) In mice, there are 2 homologs of human MGL, mMGL1 and mMGL2. Murine MGL1 shares significant sequence homology with human MGL and binds oligosaccharides with multiple terminal Gal residues including the T antigen. Interestingly, the markedly enhanced clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF in VWF−/−Asgr1−/− mice was significantly attenuated in the presence of an mMGL blocking antibody vs isotype immunoglobulin G (IgG) control antibody, respectively (α2-3 Neu-VWF t1/2 = 21.9 ± 11.8 minutes vs 9.1 ± 1.5 minutes, P < .05; and α2-3,6,8,9 Neu-VWF t1/2 = 24.4 ± 8.1 minutes vs 5.7 ± 2.1 minutes, P < .05, respectively). (E) THP1 macrophages incubated with VWF demonstrated VWF-MGL colocalization detected by Duolink-PLA, visualized as red spots via immunofluorescence microscopy. No signal was observed from cells incubated with phosphate-buffered saline (PBS) alone. (F) Plasma VWF:Ag levels were significantly elevated in MGL1−/− mice compared with wild-type (WT) littermate controls (P < .05). (G) The clearance of endogenous murine VWF in MGL1−/− mice was significantly attenuated compared with wild-type controls at all time points measured (P < .05). (H) In vivo clearance of wild-type pd-VWF in VWF−/−Asgr1−/− mice was significantly attenuated in the presence of an mMGL blocking antibody compared with isotype control IgG (t1/2 = 64.6 ± 18.4 minutes vs 42.8 ± 10.7 minutes; P < .005). A minimum of 3 mice were used per time point; data are plotted as mean ± SEM.
MGL facilitates VWF clearance in vivo. To investigate other receptors and/or cell types that modulate the enhanced clearance of hyposialylated VWF, α2-3 Neu-VWF (A) and α2-3,6,8,9 Neu-VWF (B) clearance studies in VWF−/−Asgr1−/− mice were repeated in the presence of ASOR, or following clodronate-induced macrophage depletion. The enhanced clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF was still inhibited by ASOR (blue lines) even in the absence of the AMR (α2-3 Neu-VWF t1/2 = 8.2 ± 1.4 minutes vs 16.8 ± 1.6 minutes, P < .005; and α2-3,6,8,9 Neu-VWF t1/2 = 3.7 ± 0.7 minutes vs 7.7 ± 1.3 minutes, P < .05, respectively). In addition, clodronate-induced macrophage depletion (green lines) also significantly attenuated the enhanced clearance of hyposialylated VWF (α2-3 Neu-VWF t1/2 = 8.2 ± 1.4 minutes vs 24.0 ± 1.1 minutes, P < .05; and α2-3,6,8,9 Neu-VWF t1/2 = 3.7 ± 0.7 minutes vs 9.6 ± 4.1 minutes, P < .05, respectively). Three to 5 mice were used per time point, and data are represented as mean ± SEM. (C) SPR was used to evaluate the binding of immobilized purified pd-VWF to recombinant human MGL. Dose-dependent binding were observed, with Kd (app) of 18.4 ± 3 µg/mL. (D) In mice, there are 2 homologs of human MGL, mMGL1 and mMGL2. Murine MGL1 shares significant sequence homology with human MGL and binds oligosaccharides with multiple terminal Gal residues including the T antigen. Interestingly, the markedly enhanced clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF in VWF−/−Asgr1−/− mice was significantly attenuated in the presence of an mMGL blocking antibody vs isotype immunoglobulin G (IgG) control antibody, respectively (α2-3 Neu-VWF t1/2 = 21.9 ± 11.8 minutes vs 9.1 ± 1.5 minutes, P < .05; and α2-3,6,8,9 Neu-VWF t1/2 = 24.4 ± 8.1 minutes vs 5.7 ± 2.1 minutes, P < .05, respectively). (E) THP1 macrophages incubated with VWF demonstrated VWF-MGL colocalization detected by Duolink-PLA, visualized as red spots via immunofluorescence microscopy. No signal was observed from cells incubated with phosphate-buffered saline (PBS) alone. (F) Plasma VWF:Ag levels were significantly elevated in MGL1−/− mice compared with wild-type (WT) littermate controls (P < .05). (G) The clearance of endogenous murine VWF in MGL1−/− mice was significantly attenuated compared with wild-type controls at all time points measured (P < .05). (H) In vivo clearance of wild-type pd-VWF in VWF−/−Asgr1−/− mice was significantly attenuated in the presence of an mMGL blocking antibody compared with isotype control IgG (t1/2 = 64.6 ± 18.4 minutes vs 42.8 ± 10.7 minutes; P < .005). A minimum of 3 mice were used per time point; data are plotted as mean ± SEM.
MGL is a C-type lectin receptor expressed as a homo-oligomer on antigen-presenting cells such as macrophages and dendritic cells (supplemental Figure 3).17 The carbohydrate recognition domain of MGL binds with high affinity to glycoproteins expressing terminal N-acetylgalactosamine or galactose (Gal) residues, and thus MGL can regulate glycoprotein endocytosis.18-20 MGL binding to oligosaccharide chains is attenuated by terminal sialylation.21 Importantly, given the putative role of VWF O-linked glycans in modulating clearance, MGL also recognizes the T antigen.22 In mice, there are 2 homologs of human MGL, mMGL1 and mMGL2.23 Murine MGL1 shares significant sequence homology with human MGL and has been shown to bind oligosaccharides with terminal Gal residues including the so called T antigen.23 Of note, previous studies have demonstrated that ∼70% of the O-glycans of VWF are composed of this sialylated tumor-associated T antigen structure.4,7 Interestingly, the enhanced clearance of both α2-3 Neu-VWF and α2-3,6,8,9 Neu-VWF in VWF−/−/Asgr1−/− mice was significantly attenuated in the presence of anti-mMGL1/2 inhibitory antibody, suggesting a novel role for MGL in regulating macrophage-mediated clearance of hyposialylated VWF (Figure 2D). Importantly, we observed dose-dependent binding of human pd-VWF to purified recombinant human MGL using SPR (Figure 2C). Moreover, Duolink-PLA analysis demonstrated that VWF colocalizes with MGL on the surface of THP1 macrophages, as indicated by the distinct red fluorescent dots (Figure 2E). Plasma VWF:Ag levels were significantly elevated in MGL1−/− mice compared with wild-type controls (152.6 ± 15.7% vs 100 ± 16.9%; P < .05) (Figure 2F). Furthermore, in vivo clearance of endogenous murine VWF was attenuated in MGL1−/− mice (Figure 2G), suggesting that MGL-mediated VWF clearance is important even in the presence of AMR. Finally, clearance of pd-VWF in VWF−/−/Asgr1−/− mice was attenuated in the presence of mMGL1/2 inhibitory antibody (Figure 2H). Collectively, these findings reveal MGL as a novel macrophage lectin receptor for VWF that contributes to the clearance of both wild-type and hyposialylated VWF. Further studies will be required to determine the importance of MGL compared with other recently described receptors involved in regulating VWF clearance.24 Nevertheless, the role of MGL in modulating VWF clearance has direct translational relevance in that quantitative variations in N- and O-linked sialylation have been described in patients with type 1 VWD.7,9 In addition, desialylation of VWF has been described with glycoprotein ageing in plasma25 and can also occur during infections with specific pathogens that are associated with significantly enhanced neuraminidase activity (eg, Streptococcus pneumonia).10
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nico van Rooijen of the Foundation Clodronate Liposomes (Haarlem, The Netherlands) for generously providing the liposome-clodronate.
This work was supported by a Science Foundation Ireland Principal Investigator Award (11/PI/1066, J.S.O.).
Authorship
Contribution: S.E.W., J.M.O., S.A., C.D., C.N.J., J.S., P.G.F., T.M.B., P.S., O.S., and A.C. performed experiments; S.E.W., J.M.O., S.A., C.D., C.N.J., J.S., P.G.F., T.M.B., R.J.S.P., O.S., A.C., and J.S.O. designed the research and analyzed the data; and all authors were involved in writing and reviewing the manuscript.
Conflict-of-interest disclosure: J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, and Octapharma; has served on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, and Pfizer; and has received research grant funding awards from Baxter, Bayer, Pfizer, and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: Jamie M. O’Sullivan, Department of Molecular and Cellular Therapeutics, Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland, 123 St Stephen’s Green, Dublin 2, Ireland; e-mail: jamieosullivan@rcsi.ie.
References
Author notes
S.E.W. and J.M.O. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal