Key Points
TTP patients who display persistent and severe ADAMTS13 deficiency after remission have a relapse rate of 74% during long-term follow-up.
Preemptive rituximab can decrease TTP relapses in 85% of patients with a favorable benefit-risk balance.
Abstract
Preemptive rituximab infusions prevent relapses in immune thrombotic thrombocytopenic purpura (iTTP) by maintaining normal ADAMTS13 activity. However, the long-term outcome of these patients and the potential adverse events of this strategy need to be determined. We report the long-term outcome of 92 patients with iTTP in clinical remission who received preemptive rituximab after identification of severe ADAMTS13 deficiency (activity <10%) during the follow-up. Thirty-seven patients had >1 iTTP episode, and the median cumulative relapse incidence before preemptive rituximab was 0.33 episode per year (interquartile range [IQR], 0.23-0.66). After preemptive rituximab, the median cumulative relapse incidence in the whole population decreased to 0 episodes per year (IQR, 0-1.32; P < .001). After preemptive rituximab, ADAMTS13 activity recovery was sustained in 34 patients (37%) during a follow-up of 31.5 months (IQR, 18-65), and severe ADAMTS13 deficiency recurred in 45 patients (49%) after the initial improvement. ADAMTS13 activity usually improved with additional courses of preemptive rituximab. In 13 patients (14%), ADAMTS13 activity remained undetectable after the first rituximab course, but retreatment was efficient in 6 of 10 cases. In total, 14 patients (15%) clinically relapsed, and 19 patients (20.7%) experienced benign adverse effects. Preemptive rituximab treatment was associated with a change in ADAMTS13 conformation in respondent patients. Finally, in the group of 23 historical patients with iTTP and persistently undetectable ADAMTS13 activity, 74% clinically relapsed after a 7-year follow-up (IQR, 5-11). In conclusion, persistently undetectable ADAMTS13 activity in iTTP during remission is associated with a higher relapse rate. Preemptive rituximab reduces clinical relapses by maintaining a detectable ADAMTS13 activity with an advantageous risk-benefit balance.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2210.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. Authors Karen Vanhoorelbeke and Agnès Veyradier serve as an advisor or consultant for Ablynx NV. Author Paul Coppo serves as an advisor or consultant for Alexion Pharmaceuticals, Inc., Ablynx NV, Shire, and Octapharma. Associate Editor José A. López and the remaining authors declare no competing financial interests.
Learning objectives
Describe clinical outcomes after preemptive rituximab in 92 patients with immune-mediated thrombotic thrombocytopenic purpura (iTTP) in clinical remission but with severe ADAMTS13 deficiency during follow-up
Assess adverse events after preemptive rituximab in 92 patients with iTTP in clinical remission but with severe ADAMTS13 deficiency during follow-up
Interpret ADAMTS13 conformation changes after rituximab administration and long-term outcome in patients with persistent severe ADAMTS13 deficiency after preemptive rituximab
Release date: November 15, 2018; Expiration date: November 15, 2019
Introduction
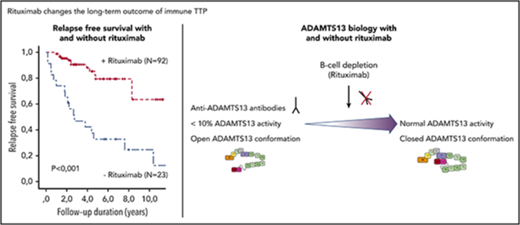
Thrombotic thrombocytopenic purpura (TTP) is a fulminant and potentially fatal disease in the absence of treatment, resulting from severe mostly immune-mediated deficiency in the von Willebrand factor–cleaving protease ADAMTS13 (ie, immune thrombotic thrombocytopenic purpura [iTTP]). Emergency treatment consists of daily therapeutic plasma exchange (TPE), usually in association with steroids, until durable remission (remission rates now >80%).1,2 Recently, the identification of a major role for anti-ADAMTS13 antibodies in iTTP pathophysiology led to associate the B-cell–depleting monoclonal antibody rituximab with the standard treatment, initially as a salvage therapy in patients with suboptimal response and now increasingly as frontline treatment. This combination allows reducing the duration of TPE and delaying relapses.1,2 Nevertheless, up to 50% of patients will experience 1 or several relapses of unpredictable severity, exposing them to death and treatment-related complications.3,4 Therefore, relapse prevention is a crucial issue. It has been reported that persistently undetectable ADAMTS13 activity in patients otherwise in remission represents a reliable early predictor of full clinical relapse.5-7 Although the natural history of ADAMTS13 activity in patients with iTTP is not completely understood, it is increasingly acknowledged that a high proportion of patients with persistently undetectable ADAMTS13 activity will experience potentially fatal relapses (38% after 1 year and 59% after 5.4 years of follow-up).5-11 It has been shown that preemptive infusions of rituximab in patients with persistently undetectable ADAMTS13 activity allow, in most of them, the rapid recovery of ADAMTS13 activity.9-11 However, this recovery may not be sustained, and a substantial number of patients require repeated rituximab infusions to maintain a detectable enzyme activity, exposing them to possible side effects. Consequently, the systematic preemptive use of rituximab to treat patients with iTTP is still debated.9-11 Therefore, it is crucial to get additional insights into the long-term outcome of these patients to draw more definitive recommendations. Moreover, it was recently reported that ADAMTS13 is in an abnormal open conformation during the acute phase of iTTP and in the physiologically closed conformation when patients are in remission (ADAMTS13 activity >50%).12 However, the pathophysiological role of ADAMTS13 conformation changes and the involved mechanisms have not been determined.
We present a large updated series of patients with iTTP treated preemptively with rituximab from the French Reference Center for Thrombotic Microangiopathies. Additionally, we studied ADAMTS13 conformation before and after preemptive rituximab administration in available patients.
Materials and methods
Patients were enrolled prospectively in our registry from January of 2012 to February of 2017 through the French Reference Center for Thrombotic Microangiopathies network (www.cnr-mat.fr). Moreover, the outcome of patients reported previously7 was updated. The diagnostic criteria for iTTP and the definitions of remission, refractoriness, exacerbation, and relapse were based on previous studies13,14 (supplemental Table 1, available on the Blood Web site). The current study focused on idiopathic iTTP; therefore, patients with iTTP associated with cancer, pregnancy, chemotherapy, or transplantation were not included. Treatment of iTTP during the acute phase was performed according to previous studies and in accordance with international guidelines.13,14 Briefly, TPE was performed daily immediately after iTTP diagnosis. The volume exchanged was 1.5 times the predicted plasma volume (standard intensive treatment) until remission, as defined by a platelet count ≥150 × 109/L for >2 days with a normal lactate dehydrogenase level. After remission, the TPE sessions were usually reduced over 3 weeks (3 sessions during the first week, 2 sessions the following week, and 1 session the last week; maintenance treatment). In the case of disease exacerbation or relapse, daily TPE sessions were resumed. Most patients received glucocorticoid therapy (prednisone, 1.5 mg/kg per day for 3 weeks; clinical features and treatment modalities are detailed in supplemental Table 2). In patients with refractory disease or exacerbation, rituximab was proposed in association with standard treatment as salvage therapy.13,14
Among the patients with durable remission from a previous TTP episode clearly identified as caused by an autoimmune mechanism, patients with severe ADAMTS13 deficiency, either persisting after clinical remission or after an initial partial or complete enzyme activity recovery, were evaluated. To assess the relapse rate after preemptive rituximab administration, only patients with >1 year of follow-up after preemptive treatment were retained, because it has been reported that relapses after rituximab administration typically occur after ≥1 year.13,15 To describe how preemptive rituximab changes iTTP history, the median number of iTTP episodes and the median cumulative incidence of annual relapses were compared before and after systematic preemptive rituximab. Finally, patients treated with preemptive rituximab were also compared with a distinct group of patients managed before the era of rituximab or in centers in which the use of preemptive rituximab was not the standard of care (ie, historical group).
ADAMTS13 activity was systematically assessed after the acute phase and then every 3 months during the entire study follow-up. In all patients with severe immune-mediated ADAMTS13 deficiency (ADAMTS13 activity <10%), but in remission, preemptive rituximab (375 mg/m2; Mabthera; Roche, Paris, France) was started in the days following the identification of a severe ADAMTS13 deficiency. The number of rituximab infusions for each course of treatment (ie, usually 1-4 per week) was left to the physician’s discretion. Premedication consisted of dexchlorpheniramine (10 mg, IV) and acetaminophen (1 g, IV) in all patients; patients who were not receiving glucocorticoids were also given methylprednisolone (30 mg, IV). Patients with persistent severe ADAMTS13 deficiency at ≥6 months following the first rituximab infusion were considered unresponsive, and their subsequent management (ie, retreatment with rituximab or alternative strategies) was left to the physician’s discretion. Patients who experienced new severe reductions in ADAMTS13 activity after recovery were retreated with rituximab, according to the same modalities.
Informed consent was obtained from all patients. This study was approved by the institutional review board of Saint-Antoine Hospital in accordance with the Declaration of Helsinki.
Evaluation of ADAMTS13 activity, anti-ADAMTS13 antibodies, and ADAMTS13 conformation
ADAMTS13 activity was assessed as reported previously.5-7 ADAMTS13 activity measurement was usually started at day 30 after the first preemptive rituximab infusion, although, in some patients, it was started only after 6 months. Thereafter, ADAMTS13 activity was systematically measured every 3 months until month 24.7 Severe ADAMTS13 deficiency was defined as undetectable ADAMTS13 activity (<10%), moderate ADAMTS13 deficiency was defined as activity between 10% and <50%, and normal ADAMTS13 was defined as activity ≥50%. In patients with undetectable ADAMTS13 activity, anti-ADAMTS13 antibodies were detected by enzyme-linked immunosorbent assay (Technozym R ADAMTS13 INH; Technoclone, Vienna, Austria), as recommended by the manufacturer. ADAMTS13 deficiency was considered acquired and immune-mediated if it was associated with anti-ADAMTS13 antibodies (threshold positivity ≥12 U/mL). It was also considered acquired in patients without detectable anti-ADAMTS13 antibodies but who recovered enzymatic activity during remission.7 ADAMTS13 conformation was determined as detailed elsewhere12 (supplemental Materials and methods).
Safety assessments
Toxicity and side effects were recorded. A severe infection was defined as an infection requiring hospitalization and/or IV antibiotics and/or resulting in death. Because of the long duration of rituximab action, an infection was considered possibly related to rituximab when occurring within the 12 months following a course of rituximab. Data collection and updating were performed as previously described.7
Statistical methods
Median values and interquartile ranges (IQRs) were determined for continuous variables. The Kruskal-Wallis test was used to compare continuous variables, and the χ2 test or Fisher exact test was used to compare binary data. The Kaplan-Meier estimator with the corresponding 95% confidence interval was used to compare survival in patients treated with preemptive rituximab infusion and in the historical group. P < .05 was considered significant.
Results
Patient population
Preemptive rituximab was administered to 116 patients with ADAMTS13 <10%. Among them, 5 were excluded from the analysis because of missing data. Another 19 patients were excluded from the analysis because of short follow-up (<12 months) after preemptive rituximab treatment. Finally, 92 patients with long-term follow-up (>12 months) were included (Table 1). All patients were in clinical and hematologic remission at the time of preemptive rituximab administration. Before the systematic use of preemptive rituximab, 37 patients experienced >1 iTTP episode (median, 3; IQR, 2-3) over 54 months (IQR, 45-82). This corresponded to a cumulative incidence of relapse of 0.33 episodes per year (IQR, 0.23-0.66).
Patient characteristics at the time of the first preemptive rituximab treatment
| Characteristics . | N = 92 . |
|---|---|
| Women | 67 (73) |
| Age, y | 42 (33.3-51) |
| Patients with previous iTTP episodes | 37 (40.2) |
| Rituximab during the acute phase | 50 (54.3) |
| Glucocorticoid therapy | 85 (92) |
| Persistence of severe ADAMTS13 deficiency following iTTP episode* | 25 (27) |
| Development of severe ADAMTS13 deficiency during follow-up† | 67 (73) |
| Anti-ADAMTS13 IgG antibody titer, U/mL | 28 (16-48) |
| Characteristics . | N = 92 . |
|---|---|
| Women | 67 (73) |
| Age, y | 42 (33.3-51) |
| Patients with previous iTTP episodes | 37 (40.2) |
| Rituximab during the acute phase | 50 (54.3) |
| Glucocorticoid therapy | 85 (92) |
| Persistence of severe ADAMTS13 deficiency following iTTP episode* | 25 (27) |
| Development of severe ADAMTS13 deficiency during follow-up† | 67 (73) |
| Anti-ADAMTS13 IgG antibody titer, U/mL | 28 (16-48) |
Data are given as median (25th-75th percentile) for quantitative variables and as n (%) for qualitative variables. Severe ADAMTS13 activity was defined as activity <10% (normal range for ADAMTS13 activity: 50%-100%). The positivity threshold for anti-ADAMTS13 immunoglobulin G (IgG) was 12 U/mL, according to the manufacturer’s instructions (Technoclone).
Eight patients received rituximab during the acute phase of the disease.
Forty-two patients received rituximab during the acute phase of the disease.
Outcomes after preemptive rituximab
Patients received 1 (n = 42), 2 (n = 15), or 4 (n = 33) rituximab infusions per course; only 1 patient received 5 infusions, and 1 received 9 infusions. The dose was 375 mg/m2 per infusion in 79 patients and 500 mg/m2 per infusion in 13 patients. Rituximab significantly decreased the median number of iTTP relapses in the whole population (0; IQR, 0-4) during the follow-up period of 35.8 months (IQR, 23.3-68), as well as the median cumulative incidence of relapses (0 episodes per year; IQR, 0-1.32) (P < .001 for both vs the prerituximab period).
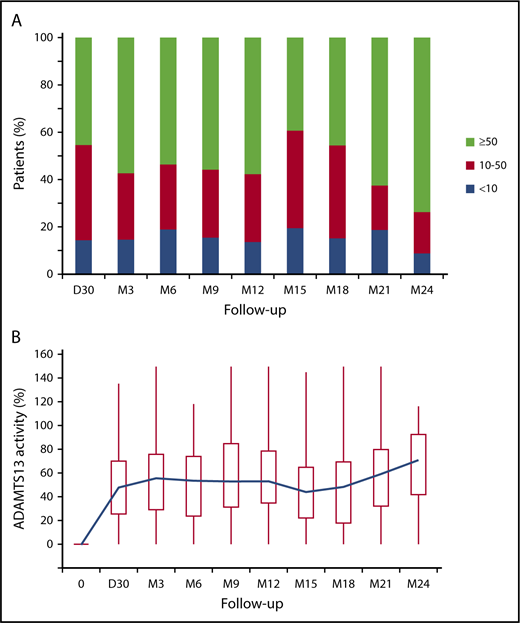
At day 30 postinfusion, ADAMTS13 activity was measured in 76 patients (83%) and was detectable (activity ≥10%) in 65 of them (86%) (Figure 1A). At 3 months postinfusion, ADAMTS13 activity was normal in 42 of 76 patients (56%), moderately decreased in 23 of 76 patients (30%), and still undetectable in the 11 patients (14%) in whom it was already severely deficient at day 30. Among these 11 patients, ADAMTS13 activity could be detected in only 2 patients at 6 months postinfusion, whereas it remained undetectable in the other 9. Among the 16 patients in whom ADAMTS13 activity was evaluated only starting at month 6 postinfusion, it was undetectable in 4; in the other 12 it was moderate (n = 6) or normal (n = 6). At any time point during the follow-up, ADAMTS13 activity was detectable in >80% of patients (Figure 1A). During the 24-month follow-up, the median ADAMTS13 activity increased until month 6 postinfusion and then progressively decreased until month 15. Then, it increased again to month 24, as a result of rituximab retreatment (Figure 1B).
ADAMTS13 activity at different time points of the study. (A) Percentage of patients with normal (≥50%, green), moderately decreased (10% to <50%, red), or undetectable (<10%, blue) ADAMTS13 activity at day 30 and at months 3, 6, 9, 12, 15, 18, 21, and 24 (>80/92 patients available) after preemptive rituximab infusion. (B) ADAMTS13 activity (%) at day 0 (time of the first preemptive rituximab administration) and at different time points after the first infusion. Box plots represent quartiles, median, and range of the laboratory measurements. The blue line represents the variation of the mean over time. D, day; M, month.
ADAMTS13 activity at different time points of the study. (A) Percentage of patients with normal (≥50%, green), moderately decreased (10% to <50%, red), or undetectable (<10%, blue) ADAMTS13 activity at day 30 and at months 3, 6, 9, 12, 15, 18, 21, and 24 (>80/92 patients available) after preemptive rituximab infusion. (B) ADAMTS13 activity (%) at day 0 (time of the first preemptive rituximab administration) and at different time points after the first infusion. Box plots represent quartiles, median, and range of the laboratory measurements. The blue line represents the variation of the mean over time. D, day; M, month.
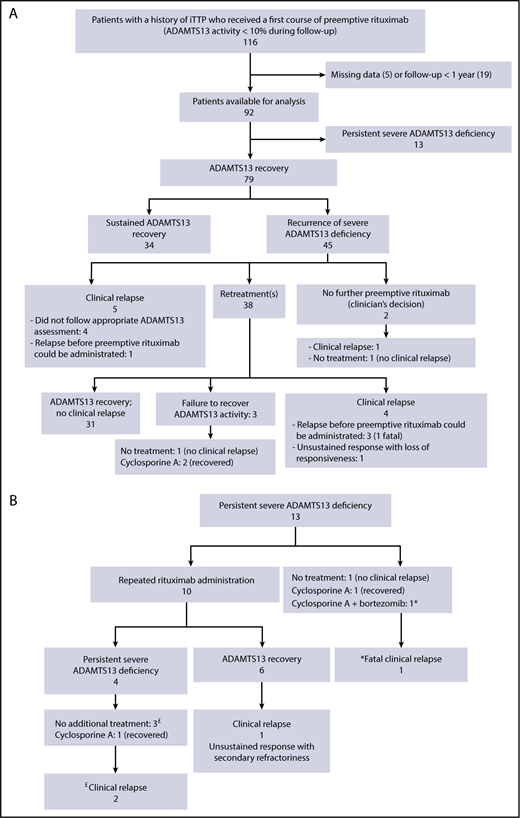
Thirty-four patients (37%) experienced sustained ADAMTS13 recovery following a single course of preemptive rituximab; these patients required no further courses of rituximab and were considered long-term responders. None reported clinical relapse or a decrease in ADAMTS13 activity during a median follow-up of 31.5 months (IQR, 18-65) after preemptive rituximab (Figure 2A).
Study flowchart. Outcome of patients with a history of iTTP who received a first course of preemptive rituximab (ADAMTS13 activity <10% during follow-up) (A). Patients who experienced a persistent severe ADAMTS13 deficiency following the first course of preemptive rituximab are detailed (B). Patients who relapsed are denoted by £ and *.
Study flowchart. Outcome of patients with a history of iTTP who received a first course of preemptive rituximab (ADAMTS13 activity <10% during follow-up) (A). Patients who experienced a persistent severe ADAMTS13 deficiency following the first course of preemptive rituximab are detailed (B). Patients who relapsed are denoted by £ and *.
At least 1 severe reduction in ADAMTS13 activity (<10% during follow-up) was observed in 45 patients (49%) after preemptive rituximab. In 5 patients, this biological relapse was associated with a clinical relapse. Among the other 40 patients, 2 received no further preemptive rituximab (clinician’s decision), whereas the other 38 received additional courses of rituximab (median, 1; IQR, 1-2) during a 39.5-month period (IQR, 27.2-70) (Figure 2A). The median time between 2 rituximab courses was 17.5 months (IQR, 12.6-25), and the length of this interval did not change over time (data not shown), suggesting no change in rituximab efficacy, despite the repeated infusions. However, the number of recurrences of severe ADAMTS13 deficiency (ie, requiring additional rituximab courses) increased with the length of the follow-up. Indeed, the 20 patients who received only 1 additional course of rituximab had a follow-up of 31 months (IQR, 22.3-66), the 13 patients who received 2 additional courses of rituximab had a follow-up of 53.3 months (IQR, 35-66), the 4 patients who received 3 additional courses of rituximab had a follow-up of 52.6 months (IQR, 45.4-65), and 1 patient who received 7 rituximab courses had a follow-up of 146 months. Only 3 patients who received 1 course of preemptive rituximab retreatment (2, 3, and 5 infusions) failed to recover a detectable ADAMTS13 activity (Figure 2A). ADAMTS13 activity was normalized with cyclosporine A in 2 of the patients. In the third patient, ADAMTS13 activity remained undetectable, but no clinical relapse was reported during a 26-month follow-up (Figure 2A). The median time between the first 2 preemptive courses of rituximab did not differ statistically between patients who received rituximab or not during the acute phase (18.4 months; IQR, 13.2-24.6 and 23.4 months; IQR, 15.2-63.4, respectively, P = .12). However, in patients who received a preemptive course of 4 rituximab infusions, the median (IQR) time to the first retreatment was longer than in those who received 2 or 1 rituximab infusion (40 months [18.1-57], 18.9 months [14.3-26], and 18.1 months [11.3-22], respectively; P = .01).
In 13 patients (14%), ADAMTS13 activity remained undetectable 6 months after the first course of preemptive rituximab (Figure 2B). Seven of these patients received rituximab during the acute phase of the disease as salvage therapy because of a suboptimal response to the standard treatment (ie, refractory disease in 4 and exacerbation in the other 3). These patients had persistently undetectable ADAMTS13 and high anti-ADAMTS13 antibody concentration after clinical remission, consistent with a primary refractoriness to rituximab. Following preemptive rituximab treatment, complete peripheral B-cell depletion was observed in 4 of the 8 patients with available data, whereas peripheral B cells were still detectable in the other 4 (range, 3-7%). Ten patients received repeated courses of rituximab (375 mg/m2), with variable modalities of administration: repeated infusions (one every 2 or 6 months for 12 to 60 months; n = 4) or a single additional course of rituximab (1 infusion [n = 1], 2 infusions [n = 3], or 4 infusions [n = 2] per course). Six of these 10 patients recovered normal ADAMTS13 activity, but the other 4 did not, despite repeated infusions (Figure 2B). Among the 3 patients who did not receive additional rituximab courses, 1 recovered ADAMTS13 activity after treatment with cyclosporine A; 1 did not show any improvement in ADAMTS13 activity, despite preemptive treatment with bortezomib and cyclosporine A, and experienced a fatal relapse; and the third patient did not receive any additional preemptive treatment and was in clinical remission, despite the persistent severe ADAMTS13 deficiency, after a 40.4-month follow-up (Figure 2B).
Altogether, 14 of 92 patients (15%) experienced a clinical relapse, leading to death in 2 of them, despite preemptive rituximab (median follow-up, 37.8 months; IQR, 20-57). Among these 14 patients with clinical relapse, 3 were unresponsive to preemptive rituximab (2 of them had repeated infusions), and 2 experienced only a transient response. Four additional patients relapsed because the ADAMTS13 assessment schedule did not comply with our recommendations (ie, every 3 months), which precluded rapid preemptive treatment after detection of severe ADAMTS13 reduction; in 4 other patients, relapse occurred rapidly after ADAMTS13 assessment and before rituximab administration. In the remaining patient, systematic treatment with preemptive rituximab was interrupted based on the clinician’s decision. In all 14 patients, relapse occurred while the patients had persistent severe ADAMTS13 deficiency (Figure 2).
ADAMTS13 conformation changes following rituximab administration
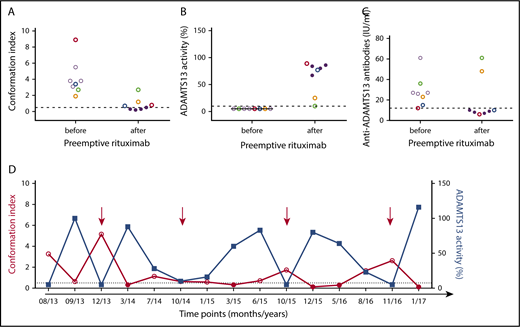
We previously showed that ADAMTS13 is in an open conformation in patients with acute iTTP (ADAMTS13 activity <10% and detectable anti-ADAMTS13 autoantibodies) and in a closed conformation in most patients with iTTP in remission.12 Here, ADAMTS13 conformation was investigated in 8 patients with undetectable ADAMTS13 activity (<10%) during remission before and after preemptive rituximab treatment (Table 2). Additionally, the ability of anti-ADAMTS13 antibodies to affect ADAMTS13 conformation was explored, because rituximab is known to deplete autoantibody production. Interestingly, before preemptive rituximab treatment, all 8 patients had an open ADAMTS13 conformation (Figure 3A). However, after preemptive rituximab treatment, ADAMTS13 conformation was closed in 4 patients (Figure 3A, black circles) who responded well to rituximab (recovered ADAMTS13 activity >50% [Figure 3B, black circles] and undetectable anti-ADAMTS13 antibody titers [Figure 3C, black circles]) (Table 2). In the remaining 2 patients who responded well to rituximab (recovered ADAMTS13 activity >50% [Figure 3B] and undetectable anti-ADAMTS13 antibodies [Figure 3C, blue and red circles]), ADAMTS13 conformation was borderline open (Figure 3A, blue and red circles) (Table 2). Conversely, in the 2 patients who were poor responders to preemptive rituximab treatment, because their ADAMTS13 activity did not fully recover (Figure 3B, green and orange circles) and anti-ADAMTS13 antibody titers remained high (Figure 3C, green and orange circles), ADAMTS13 remained open (Figure 3A, green and orange circles) (Table 2). In 1 additional patient, ADAMTS13 conformation was studied during long-term follow-up. Interestingly, although undetectable ADAMTS13 activity was associated with an opened conformation of the enzyme, administration of preemptive rituximab led systematically to a recovery of ADAMTS13 activity, along with restoration of a closed conformation (Figure 3D).
Individual data for patients with iTTP before and after the preemptive rituximab treatment
| Identification . | Delay between preemptive rituximab and sample . | ADAMTS13 activity (FRET S-VWF73), % . | Anti-ADAMTS13 IgG, IU/mL . | ADAMTS13 Ag, µg/mL . | Conformation index . |
|---|---|---|---|---|---|
| A-01 | Before | <5 | 26 | 0.44 | 3.8 |
| R-01 | 2 months | 84 | 7 | 0.78 | 0.3 |
| A-02 | Before | <5 | 27 | 0.49 | 3.1 |
| R-02 | 2 months | 80 | 8 | 0.88 | 0.3 |
| A-03 | Before | <5 | 27 | 0.26 | 5.5 |
| R-03 | 1 month | 86 | 10 | 1.00 | 0.2 |
| A-04 | Before | <5 | 12 | 0.22 | 8.9 |
| R-04 | 3 months | 89 | 6 | 1.06 | 0.8 |
| A-05 | Before | <5 | 61 | 0.23 | 3.8 |
| R-05 | 2 months | 67 | 9 | 1.2 | 0.5 |
| A-06 | Before | <5 | 15 | 0.58 | 3.4 |
| R-06 | 2 months | 77 | 10 | 1.07 | 0.7 |
| A-07 | Before | <5 | 36 | 0.34 | 2.7 |
| R-07 | 2 months | <10 | 61 | 0.48 | 2.7 |
| A-08 | Before | <5 | 23 | 0.69 | 1.9 |
| R-08 | 2 months | 25 | 48 | 0.79 | 1.2 |
| Identification . | Delay between preemptive rituximab and sample . | ADAMTS13 activity (FRET S-VWF73), % . | Anti-ADAMTS13 IgG, IU/mL . | ADAMTS13 Ag, µg/mL . | Conformation index . |
|---|---|---|---|---|---|
| A-01 | Before | <5 | 26 | 0.44 | 3.8 |
| R-01 | 2 months | 84 | 7 | 0.78 | 0.3 |
| A-02 | Before | <5 | 27 | 0.49 | 3.1 |
| R-02 | 2 months | 80 | 8 | 0.88 | 0.3 |
| A-03 | Before | <5 | 27 | 0.26 | 5.5 |
| R-03 | 1 month | 86 | 10 | 1.00 | 0.2 |
| A-04 | Before | <5 | 12 | 0.22 | 8.9 |
| R-04 | 3 months | 89 | 6 | 1.06 | 0.8 |
| A-05 | Before | <5 | 61 | 0.23 | 3.8 |
| R-05 | 2 months | 67 | 9 | 1.2 | 0.5 |
| A-06 | Before | <5 | 15 | 0.58 | 3.4 |
| R-06 | 2 months | 77 | 10 | 1.07 | 0.7 |
| A-07 | Before | <5 | 36 | 0.34 | 2.7 |
| R-07 | 2 months | <10 | 61 | 0.48 | 2.7 |
| A-08 | Before | <5 | 23 | 0.69 | 1.9 |
| R-08 | 2 months | 25 | 48 | 0.79 | 1.2 |
Patient identification, interval in months between preemptive rituximab treatment and blood sampling, ADAMTS13 activity (%), anti-ADAMTS13 antibody titers (IU/mL), and conformation index are given for 8 matched iTTP patient samples before (“A”) and after (“R”) preemptive rituximab treatment. Conformation index >0.5 indicates an open ADAMTS13 conformation.
Ag, antigen; IgG, immunoglobulin G.
ADAMTS13 conformation index and activity and anti-ADAMTS13 antibody titer before and after preemptive rituximab administration in patients with iTTP in clinical remission. ADAMTS13 conformation index (A) and activity (%) (B) and anti-ADAMTS13 antibody titers (C) before and after preemptive rituximab treatment. Closed ADAMTS13 (conformation index <0.5) is represented as filled circles, and open ADAMTS13 (conformation index >0.5) is represented as open circles. Patient samples with open ADAMTS13 conformation during remission are indicated in color. Horizontal dashed lines indicate ADAMTS13 conformation index of 0.5 with conformation index <0.5 representing closed ADAMTS13 (A), ADAMTS13 activity <10% (B), and anti-ADAMTS13 antibody titers of 12 IU/mL, with detectable anti-ADAMTS13 antibody titers when >12 IU/mL (C). (D) ADAMTS13 activity and conformation during follow-up in 1 patient treated preemptively with rituximab (red arrows). Conformation index <0.5 represents closed ADAMTS13.
ADAMTS13 conformation index and activity and anti-ADAMTS13 antibody titer before and after preemptive rituximab administration in patients with iTTP in clinical remission. ADAMTS13 conformation index (A) and activity (%) (B) and anti-ADAMTS13 antibody titers (C) before and after preemptive rituximab treatment. Closed ADAMTS13 (conformation index <0.5) is represented as filled circles, and open ADAMTS13 (conformation index >0.5) is represented as open circles. Patient samples with open ADAMTS13 conformation during remission are indicated in color. Horizontal dashed lines indicate ADAMTS13 conformation index of 0.5 with conformation index <0.5 representing closed ADAMTS13 (A), ADAMTS13 activity <10% (B), and anti-ADAMTS13 antibody titers of 12 IU/mL, with detectable anti-ADAMTS13 antibody titers when >12 IU/mL (C). (D) ADAMTS13 activity and conformation during follow-up in 1 patient treated preemptively with rituximab (red arrows). Conformation index <0.5 represents closed ADAMTS13.
In conclusion, the open conformation in iTTP patients with undetectable ADAMTS13 activity (<10%) during remission was restored (ie, closed) in the majority of iTTP patients who responded well to rituximab. Hence, these results strongly suggest an association between anti-ADAMTS13 antibodies and ADAMTS13 conformation change.
Adverse events
Adverse effects attributed to rituximab were reported in 19 patients (20.7%). They were all benign, and none led to rituximab interruption. Especially, no severe infectious complication was recorded. One patient experienced a benign nondocumented infection. Twelve patients reported moderate intolerance reactions (pruritus, rhinitis, dyspnea, nausea and vomiting, hypotension, and arrhythmia) during or within the 3 days following the first infusion. All rapidly disappeared after symptomatic treatment. Two patients experienced headache or diarrhea for few days after rituximab administration. Serum sickness (fever, fatigue, polyarthralgia, and skin rash) was observed in 4 patients 1 to 2 weeks after rituximab infusion. Acute complement consumption was documented in 2 of these 4 patients, and the outcome was systematically favorable with steroids. Rituximab was reintroduced preemptively or after a clinical relapse in 3 of these 4 patients, in association with steroids. It was well tolerated by 2 patients, whereas the other patient again experienced symptoms of serum sickness, leading to a definitive contraindication of rituximab. No case of hypogammaglobulinemia, progressive multifocal leukoencephalopathy, or Kaposi sarcoma was recorded.
Long-term outcome in patients with persistent severe ADAMTS13 deficiency
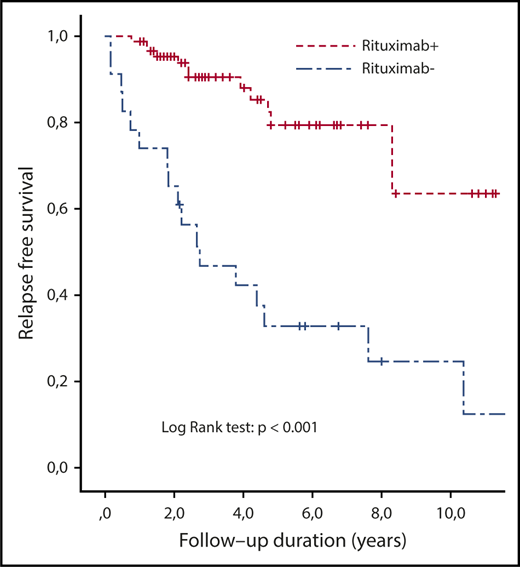
To better assess the risk of clinical relapse in patients with persistently undetectable ADAMTS13 activity, we studied 23 historical patients from our national database, who were never treated with preemptive rituximab and had persistent severe ADAMTS13 deficiency during their long-term follow-up. Their clinical features at the time of the last acute phase (particularly prognostic factors, such as age and cerebral involvement) and treatment modalities were comparable to those of the 92 patients treated with preemptive rituximab (supplemental Table 2). Their median age at the last follow-up was 41.5 years (IQR, 34.3-48), and 78% were women. The median number of iTTP episodes was 3 (IQR, 1.5-4), and 9 patients (39%) received rituximab during the acute phase of ≥1 episode. Seventeen patients (74%) had a clinical relapse (including multiple relapses in 11 of them) after a median follow-up of 7 years (IQR, 5-11), corresponding to a median cumulative incidence of relapse of 0.26 per year (IQR, 0.19-0.46). A fatal outcome was recorded for 2 patients.7 Overall, these patients experienced more relapses than did patients treated with preemptive rituximab (P < .001) (Figure 4). Among the 6 (of 23) patients who did not relapse, ADAMTS13 activity returned spontaneously to normal levels in only 2 patients who remained relapse free. The other 4 patients also remained relapse-free, despite a persistent severe ADAMTS13 deficiency, after a follow-up period of 2 to 7 years.
Kaplan-Meier survival estimates of relapse free-survival in patients with iTTP who did (n = 92) or did not (n = 23; historical group) receive preemptive rituximab. Survival between groups was compared using the Kaplan-Meier estimator.
Kaplan-Meier survival estimates of relapse free-survival in patients with iTTP who did (n = 92) or did not (n = 23; historical group) receive preemptive rituximab. Survival between groups was compared using the Kaplan-Meier estimator.
Discussion
We report more definitive results supporting the notion that preemptive infusions of rituximab prevent clinical iTTP relapses in most patients by maintaining a detectable ADAMTS13 activity. Moreover, analysis of the long-term follow-up data of historical patients with persistent severe ADAMTS13 deficiency (ie, not treated preemptively) indicates that the cumulative incidence of relapse increases dramatically with time (74% at 7 years), with fatal cases. Although 2 fatal relapses were recorded in patients who received preemptive rituximab, they were related to the failure to improve ADAMTS13 activity levels (severe ADAMTS13 deficiency unresponsive to various immunomodulatory strategies, and 1 patient experienced a fulminant relapse before beginning preemptive treatment), further supporting the need for a systematic intervention in patients with persistent severe ADAMTS13 deficiency. If, based on the present results, we hypothesize that rituximab is efficient in 85% of patients and prevents a clinical relapse in 74% of patients, the estimated number of patients to treat to prevent 1 relapse is only 1.6 [ie, 1/(0.85 × 0.74)]. Moreover, if the risk of death by relapse is 5%,5-7 the estimated number of patients to treat to prevent 1 death is 32 [ie, 1/(0.85 × 0.74 × 0.05)]. Therefore, these findings must be taken into account by practitioners in the evaluation of the benefit/risk balance when considering rituximab for patients with iTTP and persistent severe ADAMTS13 deficiency.9,10,16 Importantly, we observed no severe adverse event, despite the prolonged follow-up period (12 years for some patients). The absence of significant severe adverse events related to the use of rituximab was also reported in recently updated follow-up studies of patients with lymphoid malignancies of B-cell origin treated with rituximab in the late 1990s.17 This provides further evidence that rituximab has an acceptable long-term safety profile. The risk of progressive multifocal leukoencephalopathy, the most serious complication of rituximab, is estimated to be 1 in 25 000 overall or 1 in 500 000 for patients with autoimmune diseases (who do not have HIV or cancer).8 Additionally, we did not observe any case of Kaposi sarcoma, a complication recently reported in patients treated with rituximab for autoimmune or inflammatory systemic diseases and graft rejection.18,19 Moreover, preemptive rituximab could significantly reduce the cost of the management of patients with iTTP, because the treatment of clinical relapses includes a stay in an intensive care unit, 1 to 3 weeks of daily TPE, and, usually, multiple infusions of rituximab.13,20 Therefore, preemptive rituximab is safe and efficient and probably cost-effective in preventing iTTP relapses, even in patients who will require multiple courses of treatment.
We found that half of patients treated with preemptive rituximab required repeated courses for subsequent recurrences of ADAMTS13 deficiency. Retreated patients usually responded again to rituximab, with apparently no exhaustion of rituximab efficacy during the follow-up period. The use of rituximab during the acute phase of the disease did not significantly influence the duration of the response to the first preemptive administration. However, we observed that the interval between treatments was twice as long after a preemptive course of 4 infusions compared with a course with 1 or 2 infusions.21 We also found that half of the patients who were refractory to the first standard dose of preemptive rituximab responded to more intensive regimens that were based on those given to patients with low-grade lymphoid malignancies.22 Of note, undetectable ADAMTS13 activity at day 30 after preemptive infusion of rituximab was usually predictive of lack of response at 3 and 6 months. Similarly, patients who were unresponsive to standard preemptive rituximab usually had been unresponsive to rituximab during the acute phase of the disease (ie, ADAMTS13 activity after clinical and hematological remission remained undetectable). Consequently, refractoriness to standard doses of rituximab at such early time points suggests that more intensive treatments should be tested in an attempt to deplete anti-ADAMTS13 antibody-producing B cells more efficiently and to circumvent this apparent lack of response. Alternatively, in unresponsive patients, anti-ADAMTS13 antibodies could be produced by long-lived plasma cells, opening a possible role for antiplasma cell strategies.23
The prevalence of retreated patients in our study was substantially higher than previously reported in a smaller number of patients with more limited follow-up.7 Here, we observed that the number of recurrences of severe ADAMTS13 deficiency increased with the length of the follow-up period (ie, patients with a longer follow-up had more relapses). From these observations, we suggest that a long-term follow-up should be offered to all patients with TTP after the first acute episode, and this should include regular monitoring of ADAMTS13 activity (the optimal schedule remains to be defined). Of note, almost half of all relapses in our study, including 1 fatal case, occurred in rituximab responders in whom ADAMTS13 activity was not fully normalized. Therefore, an optimal schedule of ADAMTS13 activity assessment could further improve relapse prevention by preemptive rituximab in patients with iTTP. On the other hand, anti-ADAMTS13 antibody titers were low in all patients before the first course of preemptive rituximab administration, suggesting that, in the present study, rituximab was administered earlier than in our previous study,7 reflecting the more widespread use of this strategy in France. Therefore, this strategy, in parallel with the need to systematically assess the occurrence of connective tissue diseases,24 represents a paradigm shift in the management of iTTP, which must be considered a chronic disease. Future studies should focus on how to optimize the long-term management of these patients to improve their quality of life. This should also include the evaluation of the more recent subcutaneous formulation of rituximab for easier treatment,25 as well as the prevention of neurocognitive disorders and other disabling complications.4,26-28
Of note, in the present study, the cumulative incidence of relapse was reduced by 50% compared with our previous study (0.33 vs 0.57 episodes per year).7 This could result from the increasing use of rituximab, during the acute phase of the disease in patients who experience a suboptimal response to standard treatment, or even as frontline treatment, which has been associated with a substantial reduction in relapses.11,15
Finally, we analyzed the role of preemptive rituximab treatment and anti-ADAMTS13 antibody depletion in ADAMTS13 conformation. We found that, in most patients who responded to preemptive rituximab treatment, it normalized ADAMTS13 activity, as well as changed ADAMTS13 from the open to the closed conformation. These results suggest a role for anti-ADAMTS13 antibodies in ADAMTS13 conformation change. We could hypothesize that, by inhibiting anti-ADAMTS13 antibody production, rituximab leads to clinical remission and normal ADAMTS13 activity, together with a closed ADAMTS13 conformation. Additional studies are needed to determine whether/how anti-ADAMTS13 antibodies are implicated in opening the ADAMTS13 conformation. Moreover, it will be important to test whether ADAMTS13 conformation analysis could contribute to optimizing the monitoring of ADAMTS13 activity after preemptive treatment.
One limitation of our study is that patients were treated over different time periods (before vs after preemptive rituximab vs historical controls), raising the possibility that other changes in iTTP management could have affected the results. However, because the only difference in care between these periods was the use of preemptive rituximab, this scenario seems unlikely.
In conclusion, we report more definitive results supporting the use of preemptive rituximab in iTTP, with an advantageous risk-benefit balance. By depleting anti-ADAMTS13 antibodies, rituximab allows normalization of ADAMTS13 activity and conformation. Additional studies are needed to determine the optimal dose of rituximab and to better identify, among patients with undetectable ADAMTS13 activity, those at a higher short-term risk for relapse and who will need a more personalized preemptive strategy.29,30
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Thouzeau, S. Capdenat, S. Savigny (Laboratoire d'Hématologie, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, Paris) and S. Malot (Centre de Référence des Microangiopathies Thrombotiques, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, Paris) for technical assistance. Patients were recruited with the help of members of the Reference Center for Thrombotic Microangiopathies (“Appendix”).
This work was funded in part by a grant from the French Ministry of Health (Projet Hospitalier de Recherche Clinique, P120118, AOM12259), the National Plan for Rare Diseases of the French Ministry of Health (Direction Générale de l’Offre de Soin), and by a Horizon 2020 Marie Skłodowska-Curie Innovative Training Network grant PROFILE (675746). A.-S.S. is supported by a PhD grant (141136) from the Agency for Innovation and Entrepreneurship, Flanders, Belgium.
Authorship
Contribution: M.J. performed the cross-sectional analysis of the French Registry for Thrombotic Microangiopathies, carried out most of the phenotypical analysis, and performed the statistical analysis; P. Coppo and Y.B. designed the study, interpreted the results, wrote the manuscript, and critically reviewed the manuscript; A.-S.S., E.R., and K.V. determined the open/closed conformation of ADAMTS13, interpreted and added the data, and critically reviewed the manuscript; Y.B. also performed the statistical analysis; A.V. performed ADAMTS13 analysis and critically reviewed the manuscript; and Y.B., F.P., L.G., M. Hié, C.P., P. Poullin, A.W., S.S., C.D., A.S., S.G., Y.D., T.K., A.L., D.C., C.M., P. Perez, J.-M.H., A.C.-R., M. Hamidou, P. Cathébras, and P. Coppo enrolled patients, collected clinical and laboratory information, and critically reviewed the manuscript.
Conflict-of-interest disclosure: P. Coppo is member of the Clinical Advisory Board for Alexion, Ablynx, Shire, and Octapharma. A.V. and K.V. are members of the Clinical Advisory Board for Ablynx. The remaining authors declare no competing financial interests.
The members of the French Thrombotic Microangiopathies Reference Center are listed in “Appendix.”
Correspondence: Paul Coppo, Centre de Référence des Microangiopathies Thrombotiques, Service d’Hématologie, Hôpital Saint-Antoine, Sorbonne Université-184 rue du Faubourg Saint-Antoine, Assistance Publique-Hôpitaux de Paris, 75012 Paris, France; e-mail: paul.coppo@aphp.fr.
Appendix
The members of the French Thrombotic Microangiopathies Reference Center are: Jean-François Augusto (Service de Néphrologie, Dialyse et Transplantation, CHU Larrey, Angers); Elie Azoulay (Service de Réanimation Médicale, Hôpital Saint-Louis, Paris); Virginie Barbay (Laboratoire d’Hématologie, Centre Hospitalier Universitaire (CHU) Charles Nicolle, Rouen); Ygal Benhamou, Dominique Bordessoule (Service d’Hématologie, Hôpital Dupuytren, Limoges); Christophe Charasse (Service de Néphrologie, Centre Hospitalier de Saint-Brieuc); Anne Charvet-Rumpler, Dominique Chauveau, Gabriel Choukroun (Service de Néphrologie, Hôpital Sud, Amiens); Jean-Philippe Coindre (Service de Néphrologie, Centre Hospitalier Le Mans); Paul Coppo, Elise Corre (Service d’Hématologie, Hôpital Saint-Antoine, Paris); Yahsou Delmas, Georges Deschenes (Service de Néphrologie Pédiatrique, Hôpital Robert Debré, Paris); Alain Devidas (Service d’Hématologie, Hôpital Sud-Francilien, Corbeil-Essonnes); Antoine Dossier (Service de Néphrologie, Hôpital Bichat, Paris); Olivier Fain (Service de Médecine Interne, Hôpital Saint-Antoine, Paris); Fadi Fakhouri (Service de Néphrologie, CHU Hôtel-Dieu, Nantes); Véronique Frémeaux-Bacchi (Laboratoire d’Immunologie, Hôpital Européen Georges Pompidou, Paris); Lionel Galicier, Steven Grangé (Service de Réanimation Médicale, CHU Charles Nicolle, Rouen); Bertrand Guidet (Service de Réanimation Médicale, Hôpital Saint-Antoine, Paris); Jean-Michel Halimi, Mohamed Hamidou, Raoul Herbrecht (Service d’Oncologie et d’Hématologie, Hôpital de Hautepierre, Strasbourg); Miguel Hié, Frédéric Jacobs (Service de Réanimation Médicale, Hôpital Antoine Béclère, Clamart); Bérangère Joly (Service d’Hématologie Biologique, Hôpital Lariboisière, Paris); Tarik Kanouni, Gilles Kaplanski (Service de Médecine Interne, Hôpital la Conception, Marseille); Alexandre Lautrette, Véronique Le Guern (Unité d’Hémaphérèse, Service de Médecine Interne, Hôpital Cochin, Paris); Chantal Loirat (Service de Néphrologie Pédiatrique, Hôpital Robert Debré, Paris); Bruno Moulin (Service de Néphrologie, Hôpital Civil, Strasbourg); Christiane Mousson, Mario Ojeda Uribe (Service d’Hématologie, Hôpital Emile Muller, Mulhouse); Abdelkader Ouchenir (Service de Réanimation, Hôpital Louis Pasteur, Le Coudray); Nathalie Parquet (Unité de Clinique Transfusionnelle, Hôpital Cochin, Paris); Julie Peltier (Urgences Néphrologiques et Transplantation Rénale, Hôpital Tenon, Paris); Frédéric Pène (Service de Réanimation Médicale, Hôpital Cochin, Paris); Pierre Perez, Pascale Poullin, Claire Pouteil-Noble (Service de Néphrologie, CHU Lyon-Sud, Lyon); Claire Presne, François Provôt, Eric Rondeau (Urgences Néphrologiques et Transplantation Rénale, Hôpital Tenon, Paris); Samir Saheb, Benoît Schlemmer (Service de Réanimation Médicale, Hôpital Saint-Louis, Paris); Amélie Seguin (Service de Réanimation Médicale, Centre Hospitalier de Vendée, La Roche sur Yon, France); Aude Servais, Alain Stépanian (Laboratoire d’Hématologie, Hôpital Lariboisière, Paris); Jean-Paul Vernant (Service d’Hématologie, Hôpital la Pitié-Salpétrière, Paris); Agnès Veyradier, Cécile Vigneau (Service de Néphrologie, Hôpital Pontchaillou, Rennes); Alain Wynckel, and Patricia Zunic (Service d’Hématologie, Groupe Hospitalier Sud-Réunion, la Réunion).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal