Abstract
Antiphospholipid syndrome (APS), heparin-induced thrombocytopenia, and paroxysmal nocturnal hemoglobinuria are 3 acquired thrombophilias that carry a high risk of venous and arterial thromboembolism. Management of these conditions has largely included anticoagulation with a vitamin K antagonist after an initial period of a parenteral anticoagulant, for as long as the thrombotic risk is still present. The available evidence for the use of direct oral anticoagulants (DOACs) is limited and primarily consists of case series and cohort studies, which are summarized in this chapter. Randomized trials evaluating DOACs in patients with APS are reviewed. Further research is needed prior to widely adopting DOACs for use in these high-risk acquired thrombophilias; however, there may be selected low-risk subgroups where DOAC use is possible after careful consideration and patient discussion.
Introduction
Antiphospholipid syndrome (APS), heparin-induced thrombocytopenia (HIT), and paroxysmal nocturnal hemoglobinuria (PNH) are 3 acquired conditions with potent prothrombotic states that affect both the venous and arterial circulation and require special anticoagulant considerations. With the widespread use of direct oral anticoagulants (DOACs) for the treatment and secondary prevention of venous thromboembolism (VTE) in the general population, it begs the question: is there a role for DOACs in patients with APS, HIT, or PNH?
The pathogenesis of thromboembolic disease in APS, HIT, and PNH differs from that of the general VTE population and includes cellular- and/or complement-mediated mechanisms.1-3 There is a higher incidence of arterial thromboembolism (ATE) as well as recurrent VTE while on anticoagulation when compared with other thrombotic conditions. DOACs are convenient and may provide more stable anticoagulation compared with vitamin K antagonists (VKAs) such as warfarin, but there are possible disadvantages to their use because of their pharmacokinetic profiles. Unlike warfarin, DOACs have only 1 molecular target so could be less effective in potent thrombophilic states. Anticoagulant doses needed to prevent arterial thrombosis are higher, so DOACs may not be adequate.4 Lastly, DOACs’ short half-lives may theoretically lead to additional “breakthrough” thrombosis and anticoagulation failures. Evidence is needed before we can widely adopt the use of DOACs in these high-risk populations.
This chapter provides a basic overview of APS, HIT, and PNH and reviews the thrombotic risk in each condition. The emphasis will be on anticoagulant management, with a focus on the available evidence and current role of the DOACs.
Possibility of DOAC use: extrapolation from the cancer-associated VTE literature
Using DOACs in other prothrombotic states is theoretically possible based on evidence from the cancer-associated VTE literature. As a highly prothrombotic state, cancer and its treatment is associated with recurrent VTE despite therapeutic anticoagulation.5,6 In the recent Hokusai randomized trial, edoxaban was noninferior to low-molecular-weight heparin (LMWH) for its composite end point (recurrent VTE or major bleeding) in patients with active cancer, with fewer recurrent VTE events in the edoxaban arm.7 These data are supported by other studies showing similar outcomes in DOAC-treated cancer patients.8,9 However, there was also an increased bleeding risk among Hokusai patients who were randomized to DOACs, particularly among certain subgroups of cancer patients, highlighting the importance of disease-specific considerations and differing underlying pathophysiology.7 Thus, while the use of DOACS in other acquired thrombophilias is theoretically possible, we must look to the available disease-specific evidence to determine the optimal use of DOACs in our patients.
APS
APS and the risk of thrombosis
APS is a systemic autoimmune disorder characterized by thrombotic and/or obstetrical complications and persistently positive antiphospholipid antibodies (APLA). Autoantibodies to phospholipid-binding proteins are thought to be pathogenic through a variety of mechanisms, including interacting with the coagulation and fibrinolytic systems, monocyte and neutrophil activation, endothelial cell activation, and complement-mediated processes.1,10
The consensus-based revised Sapporo/Sydney classification criteria were initially developed for research purposes and can aid in the diagnosis of APS.1 The revised Sapporo/Sydney classification criteria require at least 1 laboratory criterion and 1 clinical criterion to define APS (Table 1). This includes (but is not limited to) VTE (pulmonary embolism or deep vein thrombosis including at unusual sites) or ATE such as stroke or myocardial infarction.11 Catastrophic APS is an aggressive variant of APS with multi–organ system involvement that includes small vessel thrombosis and can develop rapidly.12
Revised Sapporo/Sydney classification criteria for APS
| APS is present if at least 1 clinical criterion and 1 laboratory criterion are met . |
|---|
| Clinical criteria |
| 1. Vascular thrombosis |
| One or more objectively confirmed arterial, venous or small vessel thrombosis in any tissue or organ. For histopathologic confirmation, thrombosis should be present without significant vessel wall inflammation. |
| 2. Pregnancy morbidity |
| a. One or more unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation or |
| b. ≥1 premature birth of a morphologically normal neonate before the 34th week of gestation because of (1) eclampsia or severe pre-eclampsia defined according to standard definitions or (2) recognized features of placental insufficiency or |
| c. ≥3 unexplained consecutive spontaneous abortions before the 10th week of gestation, with maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes excluded |
| Laboratory criteria |
| 1. Lupus anticoagulant present in plasma on ≥2 occasions at least 12 weeks apart, detected according to the guidelines of the International Society on Thrombosis and Haemostasis |
| 2. Anticardiolipin antibody of IgG and/or IgM isotype in serum or plasma, present in medium or high titer (>40 GPL or MPL, or >99th percentile) on ≥2 occasions, at least 12 weeks apart, measured by standardized ELISA |
| 3. Anti-β2 glycoprotein 1 antibody of IgG and/or IgM isotype in serum or plasma (in titer >99th percentile) present on ≥2 occasions, at least 12 weeks apart, measured by standardized ELISA |
| APS is present if at least 1 clinical criterion and 1 laboratory criterion are met . |
|---|
| Clinical criteria |
| 1. Vascular thrombosis |
| One or more objectively confirmed arterial, venous or small vessel thrombosis in any tissue or organ. For histopathologic confirmation, thrombosis should be present without significant vessel wall inflammation. |
| 2. Pregnancy morbidity |
| a. One or more unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation or |
| b. ≥1 premature birth of a morphologically normal neonate before the 34th week of gestation because of (1) eclampsia or severe pre-eclampsia defined according to standard definitions or (2) recognized features of placental insufficiency or |
| c. ≥3 unexplained consecutive spontaneous abortions before the 10th week of gestation, with maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes excluded |
| Laboratory criteria |
| 1. Lupus anticoagulant present in plasma on ≥2 occasions at least 12 weeks apart, detected according to the guidelines of the International Society on Thrombosis and Haemostasis |
| 2. Anticardiolipin antibody of IgG and/or IgM isotype in serum or plasma, present in medium or high titer (>40 GPL or MPL, or >99th percentile) on ≥2 occasions, at least 12 weeks apart, measured by standardized ELISA |
| 3. Anti-β2 glycoprotein 1 antibody of IgG and/or IgM isotype in serum or plasma (in titer >99th percentile) present on ≥2 occasions, at least 12 weeks apart, measured by standardized ELISA |
Adapted from Miyakis et al11 with permission.
ELISA, enzyme-linked immunosorbent assay.
Lupus anticoagulant (LAC) has been consistently associated with a first episode of VTE and ATE, whereas studies report a variable thrombotic risk with anticardiolipin (aCL) according to the study design and antibody titer cutoffs used.13,14 Anti-β2 glycoprotein 1 (aβ2GP1) antibodies are likely associated with a modest risk of thrombosis, but the risk has been variably reported.15,16 While not widely available or part of the revised Sapporo/Sydney classification criteria, immunoglobulin G (IgG) aβ2GP1 antibodies specific to domain 1 have been associated with an increased risk of thrombosis.17,18
The risk of initial thrombosis is likely <1% among carriers with incidental APLA.19-21 The risk of initial thrombosis is higher when multiple APLA test results are positive and may be as high as 5% per year and 37% after 10 years for individuals with triple-positive results (positive LAC, aCL, and aβ2GP1 antibodies).22,23
Because of uncertainty around the risk of recurrent VTE in APS,24-28 Garcia et al conducted a meta-analysis in patients with prior VTE and positive APLA (LAC or aCL) compared with patients with negative APLA.29 Among 3114 patients from 8 studies, the relative risk of VTE recurrence was 1.41 (95% confidence interval [CI], 0.99-2.36) among patients with positive APLA compared with negative APLA patients, and 1.94 (95% CI, 0.84-4.46) when only unprovoked VTE events were included.29 This reported VTE risk reflects a carefully selected subgroup of patients from published studies. However, definitive conclusions cannot be made because laboratory testing was only completed at a single time point and differing antibody level cutoffs were used between studies. Recently, Kearon et al completed a prospective cohort study where the authors systematically tested for LAC, aCL, and aβ2GP1 over multiple time points in 290 patients with unprovoked VTE who had stopped anticoagulation in response to negative d-dimer testing after receiving 3 to 7 months of anticoagulation.30 Among a subgroup of patients who had persistently positive APLA on 2 occasions (consistent with the revised Sapporo/Sydney classification criteria), the rate of recurrent VTE was 13.0% per person-year, which was higher than the negative APLA group (hazard ratio, 2.7; 95% CI, 1.1-6.7; P = .03) and did not change with sex or estrogen status. The authors also found that different types of positive APLAs in the same patient on the same or different occasions had a higher recurrence risk of 21.1% per person-year, which is in keeping with previous studies.30,31 This study provides additional evidence in favor of long-term anticoagulation as well as laboratory screening in patients who present with unprovoked VTE, especially if they are otherwise deemed to be at low risk of VTE recurrence and are planning to stop anticoagulation.32-34
Anticoagulant management in APS
In 2018, the gold-standard treatment of an APS patient with VTE is still a VKA with a target international normalized ratio (INR) of 2 to 335,36 after an initial overlap with LMWH or unfractionated heparin (UFH). The available DOAC data in the APS population are limited, with recent randomized trial data showing an increased risk of thrombosis among high-risk APS patients treated with DOACs.37
Patients with APS were not excluded from the original DOAC VTE treatment trials; however, limited information is available for this patient subgroup. A small post hoc analysis of possible APS patients who received dabigatran vs VKA showed no difference in VTE recurrence rates.38
Several case series and cohort studies report recurrent thrombosis risks ranging from 0% to 75%, with lower recurrent thrombotic risks reported among larger prospective studies (Table 2).39,40 In my review of all published studies that included ≥5 patients, the pooled proportion for recurrent venous, arterial, and microthrombosis among 458 DOAC-treated patients was 9.6% (Table 2). This thrombotic risk may be higher than anticipated because of patient selection and reporting bias in small case series. However, case reports are still valuable, because they capture APS patients who had rare catastrophic events after starting DOACS.41-43
Studies reporting thrombotic outcomes in APS patients treated with DOACs
| References . | Study design . | N . | Treatment . | Follow-up (range) . | Thrombosis % (n/N) . | Recurrent thrombosis by history of ATE, % (n/N) . | Recurrent thrombosis by triple antibody positivity, % (n/N) . | ||
|---|---|---|---|---|---|---|---|---|---|
| +Hx . | −Hx . | +tAb . | −tAb . | ||||||
| 113 | Retrospective | 26 | R: 15; D: 11; initial, 6 | Mean 19 mo (8-29) | 3.8 (1/26) | 0 (0/13) | 7.7 (1/13) | 0 (0/19) | 14.3 (1/7) |
| 114 | Retrospective | 35 | R: 35 | Median 10 mo (6-24) | 0 (0/35) | — (Excluded) | 0 (0/35) | NR | NR |
| 43 | Retrospective | 8 | R: 8 | 5-365 d* | 75 (6/8) | 50 (1/2) | 83.3 (5/6) | 66.7 (2/3) | 80 (4/5) |
| 115 | Retrospective | 8 | R: 7; A: 1 | Mean 19 mo (2-36) | 0 (0/8) | 0 (0/6) | 0 (0/2) | 0 (0/1) | 0 (0/7) |
| 116 | Registry | 19 | R: 17; D: 2; initial: 2 | Mean 23.3 mo (1-84) | 31.6 (6/19)† | NR | NR | NR | NR |
| 117 | Retrospective | 23 | R: 23; initial: 3 | Median 20 mo | 4.3 (1/23) | NR | NR | NR | NR |
| 44 | RCT | 54 | R: 54 | 210 d | 0 (0/54) | — (Excluded) | 0 (0/54) | 0 (0/14) | 0% (0/40) |
| 118 | Prospective/registry | 23 | R: 23; initial: 6 | Mean 35.6 (29-40) | 26.1 (6/23) | 100 (3/3) | 15 (3/20) | 50 (1/2) | 23.8 (5/21) |
| 116,119 | Prospective‡ | 56 | R: 49; A: 3; D: 4 | Mean 22.1 mo (2-43) | 10.7 (6/56);5.8/100 patient-years | 25 (2/8)§ | 8.3 (4/48) | 25 (4/16)§ | 5 (2/40) |
| 120 | Retrospective | 24 | NR | Median 31 mo | 8.3 (2/24) | NR | NR | NR | NR |
| 121 | Retrospective | 41 | R: 29; A: 7; D: 5; initial: 14 | Median 48 mo (14-62) | 12.2 (5/41) | NR | NR | NR | NR |
| Crowther and Legault 2018 | Prospective | 82 | R: 82 | Mean 18.8 mo | 4.9 (4/82);4.0/129.8 patient-years | 20 (1/5) | 3.9 (3/77) | — (0/0) | 4.9 (4/82) |
| 37 | RCT‖ | 59 | R: 59 | Mean 569 d | 12 (7/59) | 19 (4/21) | 7.9 (3/38) | 12 (7/59) | — (0/0) |
| Pooled: 9.6 (44/458) (95% CI, 7.2-12.7) | Pooled: 19.0 (11/58) (95% CI, 10.9-30.9) | Pooled: 6.5 (19/293) (95% CI, 4.2-9.9) | Pooled: 12.3 (14/114) (95% CI, 7.5-19.6) | Pooled 10.0 (16/202) (95% CI, 4.9-12.5) | |||||
| References . | Study design . | N . | Treatment . | Follow-up (range) . | Thrombosis % (n/N) . | Recurrent thrombosis by history of ATE, % (n/N) . | Recurrent thrombosis by triple antibody positivity, % (n/N) . | ||
|---|---|---|---|---|---|---|---|---|---|
| +Hx . | −Hx . | +tAb . | −tAb . | ||||||
| 113 | Retrospective | 26 | R: 15; D: 11; initial, 6 | Mean 19 mo (8-29) | 3.8 (1/26) | 0 (0/13) | 7.7 (1/13) | 0 (0/19) | 14.3 (1/7) |
| 114 | Retrospective | 35 | R: 35 | Median 10 mo (6-24) | 0 (0/35) | — (Excluded) | 0 (0/35) | NR | NR |
| 43 | Retrospective | 8 | R: 8 | 5-365 d* | 75 (6/8) | 50 (1/2) | 83.3 (5/6) | 66.7 (2/3) | 80 (4/5) |
| 115 | Retrospective | 8 | R: 7; A: 1 | Mean 19 mo (2-36) | 0 (0/8) | 0 (0/6) | 0 (0/2) | 0 (0/1) | 0 (0/7) |
| 116 | Registry | 19 | R: 17; D: 2; initial: 2 | Mean 23.3 mo (1-84) | 31.6 (6/19)† | NR | NR | NR | NR |
| 117 | Retrospective | 23 | R: 23; initial: 3 | Median 20 mo | 4.3 (1/23) | NR | NR | NR | NR |
| 44 | RCT | 54 | R: 54 | 210 d | 0 (0/54) | — (Excluded) | 0 (0/54) | 0 (0/14) | 0% (0/40) |
| 118 | Prospective/registry | 23 | R: 23; initial: 6 | Mean 35.6 (29-40) | 26.1 (6/23) | 100 (3/3) | 15 (3/20) | 50 (1/2) | 23.8 (5/21) |
| 116,119 | Prospective‡ | 56 | R: 49; A: 3; D: 4 | Mean 22.1 mo (2-43) | 10.7 (6/56);5.8/100 patient-years | 25 (2/8)§ | 8.3 (4/48) | 25 (4/16)§ | 5 (2/40) |
| 120 | Retrospective | 24 | NR | Median 31 mo | 8.3 (2/24) | NR | NR | NR | NR |
| 121 | Retrospective | 41 | R: 29; A: 7; D: 5; initial: 14 | Median 48 mo (14-62) | 12.2 (5/41) | NR | NR | NR | NR |
| Crowther and Legault 2018 | Prospective | 82 | R: 82 | Mean 18.8 mo | 4.9 (4/82);4.0/129.8 patient-years | 20 (1/5) | 3.9 (3/77) | — (0/0) | 4.9 (4/82) |
| 37 | RCT‖ | 59 | R: 59 | Mean 569 d | 12 (7/59) | 19 (4/21) | 7.9 (3/38) | 12 (7/59) | — (0/0) |
| Pooled: 9.6 (44/458) (95% CI, 7.2-12.7) | Pooled: 19.0 (11/58) (95% CI, 10.9-30.9) | Pooled: 6.5 (19/293) (95% CI, 4.2-9.9) | Pooled: 12.3 (14/114) (95% CI, 7.5-19.6) | Pooled 10.0 (16/202) (95% CI, 4.9-12.5) | |||||
Included studies of ≥5 patients. Thrombosis outcomes included VTE, ATE, or definite microthrombosis. Possible microthrombosis (recurrent migraine in 1 patient113 and cognitive alterations/refractory headaches in 2 patients43 ) were not included in the thrombotic episodes.
A, apixaban; D, dabigatran; Hx, history; initial, initial anticoagulant treatment with a DOAC; NR, not reported; R, rivaroxaban; RCT, randomized controlled trial; tAb, triple-positive antibody status; Tmt, treatment.
Time to recurrence reported.
Included 2 patients on DOACs for primary prevention.
All patients switched to a DOAC after a minimum of 3 months and d-dimer <500 ng/mL.
One patient with a recurrent VTE had both a history of ATE and triple antibody positivity.
Reported based on an on-treatment per protocol analysis.
Two of the largest studies published in low-risk APS populations have reported reassuring VTE recurrence rates with DOAC use. An open-label trial evaluated 116 patients randomized to rivaroxaban or warfarin for secondary VTE prevention with a primary surrogate outcome of percentage change in endogenous thrombin potential. Patients with ATE or recurrent VTE were excluded. The endogenous thrombin potential was higher in the rivaroxaban group than in the warfarin group and did not reach the set non-inferiority threshold of <20% difference. Although underpowered for clinical events, it is reassuring that there were no thrombotic events or major bleeding reported after 210 days in 54 APS patients who received rivaroxaban.44 A multicenter prospective cohort of 82 APS patients with a history of VTE were treated with rivaroxaban for secondary VTE prevention. No patients were triple positive, and 5 patients had a history of ATE. There were 4 thromboembolic events (2 cerebrovascular and 2 VTE) in 129.8 patient-years of follow-up, a rate similar to previous studies evaluating warfarin35,36,45 (M. Crowther and K. Legault, written communication, 17 April 2018).
Unfortunately, there is an unacceptably high thrombotic rate reported among high-risk APS patients treated with DOACs. TRAPS (Trial on Rivaroxaban in AntiPhospholipid Syndrome) is a multicenter trial that evaluated rivaroxaban versus warfarin in 120 triple-positive patients with APS.37 In the intention-to-treat analysis, there were significantly more thrombotic events in the rivaroxaban arm (14%) compared with the warfarin arm (0%), leading to early trial termination. There were 7 (12%) arterial events, including among patients with only a history of VTE.37 The ASTRO-APS (Apixaban for Secondary Prevention of Thrombosis Among Patients with Antiphospholipid Syndrome) is an open-label randomized trial evaluating apixaban vs usual care for prevention of recurrent thrombosis after at least 6 months of initial anticoagulation (www.clinicaltrials.gov identifier NCT02295475). After enrolling the first 25 patients, a preplanned Data Safety Monitoring Board review recommended an increase from apixaban 2.5 mg twice daily to apixaban 5 mg twice daily. After a higher than expected rate of stroke in 5 subsequent patients, an unplanned Data Safety Monitoring Board review recommended changing the enrollment criteria to exclude patients with prior arterial thrombosis and to obtain brain magnetic resonance imaging prior to enrollment to exclude stroke or white matter changes disproportionate for age.46
Management summary
While some of the data in lower-risk APS patients have been reassuring, recent and ongoing trials have reported an increased rate of thrombosis among APS patients receiving DOACS, particularly those with previous arterial disease or triple positivity. Based on the available evidence, we should be extremely cautious when using DOACs in patients with APS.
A recent guidance statement from the 14th International Congress on Antiphospholipid Antibodies Task Force recommends that warfarin remain the current standard of care.47 DOACs may be considered when there is a known VKA allergy/intolerance or poor anticoagulant control. At the time of publication, there were not enough data for the task force to recommend DOACs in high-risk scenarios such as those with recurrent VTE while on anticoagulation or for APS-related arterial thrombosis.47
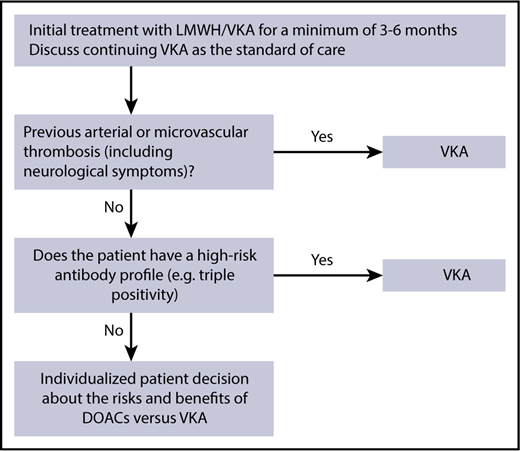
I recommend long-term anticoagulation with a VKA to APS patients as the current standard of care, unless contraindications are present such as thrombocytopenia (platelet count <50 × 109/L). Based on the available evidence, I would avoid the use of DOACs in known high-risk subgroups (eg, triple positivity or a history of arterial disease; Figure 1). There may be a role for DOACs for secondary VTE prevention in a carefully selected subgroup of low-risk APS patients, such as those with a single unprovoked VTE and low-risk antibody profile and no history of arterial thrombotic disease or neurological symptoms possibly related to small-vessel arterial disease (Figure 1). However, given the limited evidence and possible risk of arterial thrombosis, a detailed patient discussion outlining the risks and benefits is warranted. Unlike the general VTE population where dose reduction of DOACs may be an option after 6 months of initial anticoagulation,48,49 in APS patients, I would follow the product monographs and not recommend dose reduction of DOACs due to the absence of data and potential for recurrent thrombosis. Further research is still needed.
Other management scenarios in APS
ATE
Recurrent breakthrough thrombosis
If an APS patient had recurrent VTE while on therapeutic anticoagulation with warfarin (INR 2-3), I would use a short course of therapeutic LMWH until clinical improvement (eg, 3-4 weeks) and add aspirin to long-term VKA therapy. Alternative options for managing recurrent thrombosis on therapeutic anticoagulation include targeting a higher INR of 3 to 4, choosing an alternative long-term anticoagulant such as LMWH, or adding agents such as hydroxychloroquine or statins based on limited evidence.1
Catastrophic APS
Prophylaxis in high-risk settings
Patients with APS may require additional prophylaxis in high-risk settings such as pregnancy or around surgery. In pregnant patients with a previous VTE, I recommend intermediate to therapeutic doses of LMWH during pregnancy.52 In APS patients with past ATE, I recommend therapeutic doses of LMWH in pregnancy, with or without aspirin, based on the clinical scenario. Postpartum, APS patients with past thrombosis should resume LMWH until transitioned back to warfarin, with a LMWH dose that takes into account the initial bleeding risk postpartum and a patient’s individual thrombotic risk. Among patients with obstetrical APS with no past thrombosis, the risk of VTE during pregnancy is reassuringly low, and antepartum LMWH would not be needed.53 The use of antepartum LMWH and aspirin to prevent recurrent pregnancy loss is based on studies that report mixed results and have variable definitions of APS, so a patient discussion about the risks and benefits is needed.54-56 I recommend postpartum prophylactic-dose LMWH for obstetrical APS patients with high-risk antibody profiles (eg, titers >40 GPL/MPL or multiple antibody positivity) or if additional risk factors such as systemic lupus erythematosus are present and then have a preferences- and values-based patient discussion about prophylaxis for APS patients with lower-risk features.53
There are limited data for the use of anticoagulant “bridging” among APS patients when their anticoagulation is held in the perioperative setting. A retrospective cohort of 416 patients on warfarin for past VTE reported a low VTE incidence (0.32%) with minimal to no use of LMWH, and over 38% of patients tested had a high-risk thrombophilia present that included LAC or aCL positivity.57 For APS patients with past thrombosis, I typically use prophylactic doses of LMWH before and after surgery instead of therapeutic doses in order to minimize bleeding risk. Postoperative bleeding from anticoagulation can be associated with thrombosis, particularly when anticoagulants are held for a prolonged period of time due to bleeding complications.57,58
HIT
HIT and the risk of thrombosis
HIT is a “clinicopathologic” syndrome with clinical manifestations that are supported by serological and functional laboratory assays. HIT typically presents with a platelet count drop ≥50% ∼5 to 10 days after UFH/LMWH exposure (≤1 day if recent heparin exposure) and is associated with VTE and ATE and less commonly limb gangrene, heparin-induced skin lesions, and anaphylactoid reactions to heparin.2,59,60 VTE is more common than ATE (4:1), and unusual site thrombosis is possible including adrenal hemorrhage from presumed adrenal vein thrombosis.61,62 IgG antibodies recognize platelet factor 4 (PF4)/heparin complexes on the surface of platelets, which can activate platelets, monocytes and endothelial cells and lead to clinical HIT in a proportion of cases.2,63 There is an imbalance between pro- and anticoagulants, and the addition of warfarin in acute HIT can lead to venous limb gangrene because of an acquired protein C deficiency.64-66 Several variants of HIT have been described where anti-PF4 antibodies recognize PF4/polyanion complexes without heparin present and include spontaneous HIT (development in the absence of heparin), persistent HIT (where HIT takes several weeks to recover), and delayed-onset HIT (where HIT begins or worsens after stopping heparin).67
The clinical 4T score helps clinicians recognize possible HIT, where an intermediate or high probability 4T score ≥4 requires further laboratory testing68 (Table 3). Antibody-based enzyme immunoassay (EIA) testing is highly sensitive and can help to rule out HIT if the result is negative. Functional testing such as the serotonin release assay (SRA) is sensitive and specific and can confirm HIT if the result is positive and the clinical picture fits.59,68-70 If a patient has an intermediate to high pretest probability of HIT, then a positive EIA test result with a high optical density can limit SRA testing due to improved EIA specificity.71,72
4T score HIT clinical scoring system
| . | 2 points . | 1 point . | 0 points . |
|---|---|---|---|
| Thrombocytopenia | Platelet count fall >50% and platelet count nadir ≥20 × 109/L | Platelet count fall 30-50% or platelet count nadir 10-19 × 109/L | Platelet count fall <30% or platelet count nadir < 10 × 109/L |
| Timing of platelet count fall | Clear onset of thrombocytopenia 5-10 d after heparin administration or platelet count fall ≤1 d with prior heparin exposure within 30 d | Timing is consistent with a day 5-10 platelet count fall but is not clear (eg, missing platelet counts), onset after day 10, or fall ≤1 d with prior heparin exposure 30-100 d ago | Platelet count fall is <4 d without recent heparin exposure |
| Thrombosis or other sequelae | Confirmed evidence of a new thrombosis, skin necrosis (lesions at heparin injection site, or acute systemic reaction after IV unfractionated heparin bolus | Suspected (but not proven) thrombosis, progressive or recurrent thrombosis, or nonnecrotizing skin lesions | None |
| Other causes of thrombocytopenia | None apparent | Possible | Definite |
| . | 2 points . | 1 point . | 0 points . |
|---|---|---|---|
| Thrombocytopenia | Platelet count fall >50% and platelet count nadir ≥20 × 109/L | Platelet count fall 30-50% or platelet count nadir 10-19 × 109/L | Platelet count fall <30% or platelet count nadir < 10 × 109/L |
| Timing of platelet count fall | Clear onset of thrombocytopenia 5-10 d after heparin administration or platelet count fall ≤1 d with prior heparin exposure within 30 d | Timing is consistent with a day 5-10 platelet count fall but is not clear (eg, missing platelet counts), onset after day 10, or fall ≤1 d with prior heparin exposure 30-100 d ago | Platelet count fall is <4 d without recent heparin exposure |
| Thrombosis or other sequelae | Confirmed evidence of a new thrombosis, skin necrosis (lesions at heparin injection site, or acute systemic reaction after IV unfractionated heparin bolus | Suspected (but not proven) thrombosis, progressive or recurrent thrombosis, or nonnecrotizing skin lesions | None |
| Other causes of thrombocytopenia | None apparent | Possible | Definite |
The scoring system is categorized as low probability (0-3 points), intermediate probability (4-5 points), or high probability (6-8 points). Adapted from Lo et al59 with permission.
Historically, the risk of thrombosis was 38% to 53% in the first 30 days of HIT with a high mortality rate when another anticoagulant was not initiated.62,73 In a recent retrospective study across US centers that evaluated patients until hospital discharge or day 45, the thrombotic risk was ∼20% among patients with a positive EIA test result despite the majority being on a nonheparin anticoagulant.74
Anticoagulant management in HIT
Several possible anticoagulant options exist for treatment of both HIT with thrombosis (HIT-T) or without thrombosis (isolated HIT). Argatroban and danaparoid are approved for the treatment of HIT, and bivalirudin is approved for treatment of HIT for patients undergoing percutaneous coronary intervention. Danaparoid is not available in the United States. Fondaparinux and desirudin are “off label” for the treatment of HIT but have data to support their use.
There is reasonably strong data to support the use of fondaparinux in the treatment of HIT. Fondaparinux is an indirect factor Xa inhibitor that has proven efficacy in the treatment of other conditions such as VTE and acute coronary syndromes. Fondaparinux is given by subcutaneous injection and does not affect the PTT and may provide more stable dosing in severe HIT where coagulation parameters can be abnormal at baseline. The development of heparin antibodies that cross-react to fondaparinux is exceedingly rare (3 cases reported)75-77 ; autoimmune HIT or an alternative diagnosis should be considered if the platelet count does not recover after fondaparinux is initiated.
There are reports of 95 patients with likely HIT who received fondaparinux and have not had a new or progressive thrombosis.78-85 It is possible that this group represents a selected population where clinicians were comfortable using nonstandard treatment. Reassuringly, there was no difference between the thrombotic risk reported in 133 patients treated with fondaparinux (16.5%) compared with those treated with argatroban and danaparoid (21.4%) when patients were matched using propensity scoring for age, sex, creatinine, 4T score, and comorbidity index.86 Table 4 reviews drug characteristics of fondaparinux and other standard HIT treatments.
Parental anticoagulants for the management of HIT
| . | Indirect factor Xa inhibitors . | Direct thrombin inhibitors . | |||
|---|---|---|---|---|---|
| Fondaparinux . | Danaparoid . | Argatroban . | Bivalirudin . | Desirudin . | |
| Administration | Subcutaneous injection | IV infusion or subcutaneous injection | Continuous IV infusion | Continuous IV infusion | Subcutaneous injection |
| Clearance (half-life) | Renal (17 h) | Renal (24 h) | Hepatobiliary (40-50 min) | Renal/enzymatic (25 min) | Renal (2 h) |
| INR interference | No | No | Yes | Yes | Possible |
| Drug monitoring | None* | Anti-Xa activity* | PTT | PTT | None |
| Reversal agent | None† | None† | None | None | None |
| Other | Caution with renal impairment. Contraindicated with CrCl <30 mL/min | Caution with renal impairment; not available in the United States | Caution with liver dysfunction; consider dose reduction in critically ill patients | Evidence for use available in cardiac surgery and PCI | Caution with renal impairment |
| . | Indirect factor Xa inhibitors . | Direct thrombin inhibitors . | |||
|---|---|---|---|---|---|
| Fondaparinux . | Danaparoid . | Argatroban . | Bivalirudin . | Desirudin . | |
| Administration | Subcutaneous injection | IV infusion or subcutaneous injection | Continuous IV infusion | Continuous IV infusion | Subcutaneous injection |
| Clearance (half-life) | Renal (17 h) | Renal (24 h) | Hepatobiliary (40-50 min) | Renal/enzymatic (25 min) | Renal (2 h) |
| INR interference | No | No | Yes | Yes | Possible |
| Drug monitoring | None* | Anti-Xa activity* | PTT | PTT | None |
| Reversal agent | None† | None† | None | None | None |
| Other | Caution with renal impairment. Contraindicated with CrCl <30 mL/min | Caution with renal impairment; not available in the United States | Caution with liver dysfunction; consider dose reduction in critically ill patients | Evidence for use available in cardiac surgery and PCI | Caution with renal impairment |
CrCl, creatinine clearance; PCI, percutaneous coronary intervention.
Some centers monitor fondaparinux using fondaparinux-specific anti-Xa activity. Some centers do not routinely monitor danaparoid, particularly in patients with normal renal function.93
Andexanet alfa is a reversal agent for direct and indirect Xa inhibitors but has not been studied or approved for use in HIT or with reversal of fondaparinux or danaparoid. Given the potential risk of ischemic events with andexanet alfa, caution in HIT is needed.
The DOACs are attractive for use in HIT because they have a quick onset of action, do not lower protein C levels, and are not known to cause antibodies. Linkins et al87 completed a prospective study of 12 HIT patients treated with rivaroxaban. The thrombotic rate was 4.5% (95% CI, 0% to 23%) and included 1 patient with an extension of a line-associated upper extremity VTE that was treated with catheter removal and rivaroxaban continuation. One patient had worsening of bilateral lower limb arterial thrombosis requiring amputation while on rivaroxaban. Subsequently, Warkentin et al88 reported 16 additional HIT patients who were treated with rivaroxaban for HIT-T (all were VTE events) or isolated HIT. There were no new thrombotic events, and no major bleeding, limb amputation, or death occurred in 3 months of follow-up. Warkentin et al completed a systematic review of probable HIT patients (4T score ≥4 and detection of HIT antibodies or 4T score ≥6) treated with DOACs prior to platelet recovery, and the risk of new thrombosis was 2.6% (2/81).88,89 There were 11 additional HIT patients who transitioned to a DOAC after platelet count recovery with no new thrombosis and 1 major bleeding event in a patient with known varices.88 The limitation of these studies included a selected patient population where DOACs were deemed appropriate, and few HIT patients had an initial ATE.
Management summary
Because of the high risk of thrombosis, once HIT is suspected based on clinical suspicion (eg, 4T score ≥4), then heparin should be stopped and a therapeutic nonheparin anticoagulant started until confirmatory laboratory testing is completed. If I have decided to send off laboratory testing but clinically think the patient has a relatively low likelihood of HIT and am concerned for bleeding from another cause of thrombocytopenia, then I use prophylactic doses of fondaparinux (2.5 mg subcutaneously daily) until confirmatory test results come back, a practice that is based on expert opinion and limited evidence.69,86,90
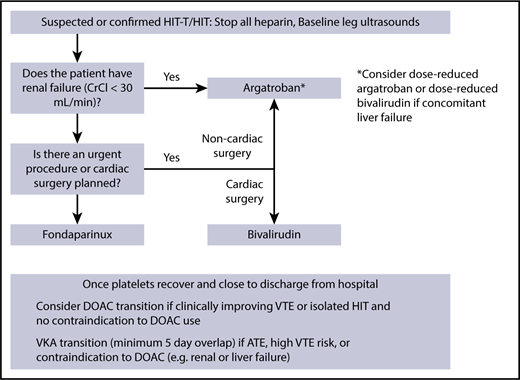
While different HIT guidelines consistently recommend starting a nonheparin anticoagulant once HIT is suspected, the choice of anticoagulant varies according to the guideline (differences outlined by Wang et al91 ). My current practice is to use therapeutic-dose fondaparinux in the acute setting of HIT-T and isolated HIT; if the patient has renal failure or requires an urgent procedure, then I use argatroban, and if the patient is undergoing cardiac surgery, then I prefer to use bivalirudin (Figure 2). It is my practice to screen all positive HIT patients with bilateral leg ultrasounds given the high risk of thrombosis. I would avoid inferior vena cava filter placement, which can be highly prothrombotic in HIT.92 I have not been using DOACs in the upfront setting, as this is the most prothrombotic period that includes ATE,62,74 and many of hospitalized patients cannot tolerate oral anticoagulation. When the patient is closer to discharge from hospital, I consider switching to a DOAC after a careful patient discussion, particularly when thrombosis is absent or if their VTE is clinically improving. If I am worried about progressive or recurrent VTE, or if the patient had an ATE, then I would overlap the nonheparin anticoagulant with VKA for a minimum of 5 days once the platelet count is >150 × 109/L because of the risk of hypercoaguability from acquired protein C deficiency from VKA.
I would treat HIT-T with anticoagulation for 3 months, because it is a provoked VTE. While controversial,74,93 I treat patients with isolated HIT for 3 months based on the fact that other known thrombotic risk factors extend outward to 3 months and HIT antibodies can be present for ∼100 days (functional test, median 50 days; EIA test, median 85-90 days).60,94 The majority of guidelines recommend 4 weeks duration of anticoagulation in isolated HIT.91 If there were risk factors for bleeding in a patient with isolated HIT, then I would stop anticoagulation at ∼4 weeks.62,73
Other management scenarios in HIT
Future anticoagulation use
For patients with confirmed HIT who require anticoagulation in the future, I recommend a nonheparin anticoagulant such as fondaparinux or a DOAC depending on the clinical scenario.
Cardiac surgery
The ACCP guidelines recommend the use of bivalirudin over other nonheparin anticoagulants or heparin plus antiplatelet agents for HIT patients requiring cardiac surgery.95 When possible, cardiac surgery should be delayed until HIT test results are negative. If it is not possible to delay surgery, then an alternative anticoagulant should be used if the EIA and SRA test results are still positive. The evidence for bivalirudin is based on prospective cohort studies in HIT patients and randomized controlled trial data in non-HIT patients,95 with protocols available on and off bypass.96,97 If the positive functional SRA test result subsequently becomes negative, then short exposure to heparin during the procedure is possible, with a nonheparin anticoagulant used postoperatively.93 Plasma exchange to remove HIT antibodies or IV immune globulin are alternative strategies that have been reported in positive HIT patients who are refractory to standard therapy or require urgent surgery.98-100
PNH
PNH and the risk of thrombosis
PNH is a rare “orphan” disease that affects 1 to 2 patients in 1 million and is characterized by intravascular hemolysis, thrombosis, immune-mediated bone marrow failure, and other symptoms related to smooth muscle dysfunction. A somatic mutation in the PIGA (phosphatidylinositol glycan complement class A) gene in hematopoietic stem cells leads to the absence of glycosylphosphatidylinositol (GPI) anchor proteins on the surface of progeny blood cells. Two important GPI-linked complement regulatory proteins, CD55 and CD59, are reduced or absent on red blood cells, which leads to complement-mediated hemolysis. The pathophysiology of thrombosis is multifactorial due to increased procoagulant microparticles, endothelial cell damage from free hemoglobin, nitric oxide depletion, complement-mediated platelet, neutrophil and monocyte activation, and absence of other GPI anchor proteins that affect the coagulation and fibrinolytic system.3,101
The cumulative thrombotic risk in PNH is ∼20% to 30% over 10 years and is associated with a high mortality rate.102-104 Patients with a large PNH granulocyte clone (>50%-60%) have a higher thrombotic risk (35%-54%) than those with a smaller clone (6%-17%).104-106 VTE is more common than ATE and includes a high proportion of unusual site thrombosis, including mesenteric and hepatic vein thrombosis.107,108 Approximately 5% to 10% of PNH patients will present with thrombosis.103,104 Screening all patients with unprovoked VTE for PNH is unnecessary and should be reserved for those presenting with unusual site thrombosis or atypical features such as hemolysis or anemia.109 The incidence of HIT is reportedly increased among PNH patients secondary to increased platelet activation with induced release of PF4.110
The introduction of effective complement blockade with eculizumab, a humanized monoclonal antibody against complement protein C5, has decreased the risk of thrombosis in patients with PNH. In a pooled analysis of 3 extension trials that enrolled 195 patients with PNH and clinically meaningful hemolytic disease (history of VTE/ATE in 32% of participants; anticoagulant use in 56% of participants), the overall thrombotic rate was reduced from 7.37% per person-year to 1.07% per person-year. Among only the patients on anticoagulation, the thrombotic rate was reduced from 10.61% per person-year to 0.62% per person-year.107 There are only a small number of reports of patients who have successfully stopped anticoagulation after their disease was controlled on eculizumab.111
Anticoagulant management in PNH
In a patient with a new VTE or ATE, I recommend starting LMWH and transitioning to a VKA. I would treat for a minimum of 3 to 6 months and as long as the underlying disease is active, unless a contraindication such as significant thrombocytopenia (platelet count <50 × 109/L) is present. What to do for patients with previous thrombosis who have stable disease on eculizumab is still unknown and warrants a detailed patient discussion about the risks and benefits of anticoagulation.110 Given the lack of evidence, I typically recommend continuing some form of anticoagulation but acknowledge the importance of patient preference and the possibility of stopping anticoagulation.
DOAC use in PNH is an “evidence-free zone.” There is 1 published case report of a PNH patient with a history of cerebral vein thrombosis who was switched to rivaroxaban after the patient's hemolysis stabilized on eculizumab.112 Based on extrapolated data and a patient discussion, I would consider a DOAC only for secondary VTE prevention if the patient had controlled disease on eculizumab. I would not use a DOAC if there was recurrent VTE, past ATE, or unusual site thrombosis or if the patient’s underlying PNH was uncontrolled given the lack of evidence and possibility of harm.
Other management scenarios in PNH
Prophylaxis use
Because eculizumab has greatly mitigated the risk of thrombosis in PNH, I would not recommend primary prophylaxis in patients who are on eculizumab. Among PNH patients who are not on eculizumab, the role of primary prophylaxis is controversial and must be balanced against the risk of bleeding.110 In situations that carry additional thrombotic risk, such as in pregnancy or in the perioperative setting, prophylactic anticoagulation with LMWH may be warranted with dosing that reflects bleeding risk (thrombocytopenia) and thrombotic risk (status of underlying disease).
Conclusion
Based on limited data, DOAC use may be possible in a carefully selected subgroup of low-risk patients with APS, HIT, and PNH, especially after an initial treatment course of LMWH/VKA (APS and PNH) or a nonheparin anticoagulant such as fondaparinux (HIT). DOACs should generally be avoided in high-risk subgroups, including those with past ATE, recurrent VTE, or evidence of active underlying disease (eg, triple positivity in APS, thrombocytopenia in HIT, or active hemolysis/large clone in PNH) until further research is completed.
Authorship
Contribution: L.S. completed the data extraction and data analysis and wrote the manuscript.
Conflict-of-interest disclosure: L.S. has received research funding and from CSL Behring and honoraria from Leo Pharma. Off-label drug use: None disclosed.
Correspondence: Leslie Skeith, Room C210, Foothills Medical Centre, 1403 29th St NW, Calgary, AB T2N 2T9, Canada; e-mail: laskeith@ucalgary.ca.
REFERENCES
Author notes
This article was selected by the Blood and Hematology 2018 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2018. It is reprinted in Hematology Am Soc Hematol Educ Program. 2018;2018:439-449.