Key Points
Pomalidomide-cyclophosphamide-dexamethasone in first relapse after exposure to lenalidomide and bortezomib is efficacious and safe.
Salvage pomalidomide-cyclophosphamide-dexamethasone can be a bridge for delayed autologous stem cell transplantation.
Abstract
It is important to have an effective therapy for patients with multiple myeloma (MM) at first relapse, particularly if an autologous stem cell transplant (ASCT) is considered at this stage. This multicenter, phase 2 trial evaluated the efficacy and safety of weekly oral pomalidomide-cyclophosphamide-dexamethasone (PCD) in patients with MM in first relapse after treatment with lenalidomide-bortezomib-dexamethasone (RVD). All patients had received RVD as induction and consolidation therapy, plus lenalidomide maintenance for 1 year (arm A). Half had also received an ASCT after induction (arm B). At MM relapse, all patients received 4 oral cycles of pomalidomide 4 mg (days 1-21), cyclophosphamide 300 mg (days 1, 8, 15, and 22), and dexamethasone 40 mg (days 1-4 and days 15-18 of a 28-day cycle; PCD). Responding patients in arm A underwent ASCT and received 2 additional cycles of PCD, whereas those in arm B received 5 cycles of PCD. All patients received pomalidomide-dexamethasone maintenance until disease progression. Primary end point was partial remission or better after the initial 4 cycles of PCD. Responses were obtained in 82/97 (85%) patients evaluated: complete remission (n = 1; 1%), very good partial remission (n = 32; 33%), and partial remission (n = 49; 51%). Three patients (3%) had stable disease, and 6 (6%) had disease progression (6 response failures). Forty-five (94%) of the 48 patients in arm A underwent planned ASCT. PCD was effective therapy after first relapse with RVD. After 4 cycles, the rate of partial remission or better was 85%, and 94% of planned ASCTs were performed. Toxicity was mostly hematologic and manageable. This trial was registered at www.clinicaltrials.gov as #NCT02244125.
Introduction
The introduction of immunomodulatory drugs (IMiDs; thalidomide, lenalidomide, pomalidomide) and proteasome inhibitors (bortezomib, carfilzomib) as first-line therapy and after relapse has improved response rates and survival in patients with multiple myeloma (MM).1 However, MM remains incurable, and the majority of patients relapse and become refractory to available therapies. There are currently 4 approved lenalidomide-based and 2 proteasome inhibitor-based combinations for patients relapsing after 1 to 3 previous lines of therapy. Lenalidomide plus dexamethasone has been combined with carfilzomib,2 ixazomib,3 elotuzumab,4 and daratumumab.5 Bortezomib plus dexamethasone has been combined with daratumumab,6 and carfilzomib has been associated with dexamethasone.7 In patients refractory to bortezomib (Velcade) and lenalidomide (Revlimid), outcomes are poor, with median progression-free survival (PFS) of 5 months and overall survival (OS) of 15 months.8 The treatment options for these patients are limited, and current triplet approvals in relapse MM either were excluded or had low numbers of patients coming off lenalidomide therapy early in their disease, and therefore not specifically addressing this patient population. The choice of treatment at relapse is based on age and comorbidities, the efficacy and toxicity of previous treatments, the duration of previous remission, and the circumstances of relapse. Pomalidomide has a higher anti-MM potential than thalidomide and lenalidomide.9 In combination with dexamethasone, it is highly effective in relapsed/refractory patients, especially after lenalidomide exposure. The overall response rate (partial remission [PR] or better) with pomalidomide plus low-dose dexamethasone in patients with late-stage relapsed MM (median of 5 previous lines) who had previously received bortezomib-lenalidomide was 34%.10-13 Alkylating agents (melphalan, cyclophosphamide) are also standard treatments for MM.
A new drug combination consisting of pomalidomide-cyclophosphamide-dexamethasone (so-called pomalidomide-cyclophosphamide-dexamethasone [PCD] association) is currently under investigation. Palumbo et al described various dosage schedules of pomalidomide with daily cyclophosphamide,14 whereas Baz et al established the maximum tolerable dose of pomalidomide with weekly cyclophosphamide-dexamethasone.15 These 2 groups published encouraging results with this completely oral, triple combination.14,15
We carried out a prospective analysis of PCD in 100 patients relapsing after first-line treatment with lenalidomide-bortezomib-dexamethasone (RVD combination), with or without an autologous stem cell transplant (ASCT), in the Intergroupe Francophone du Myélome (IFM) 2009/Dana Farber Cancer Institute (DFCI) trial.16 Overall, patients were fitter and at an earlier stage of relapsed disease (1 previous line of treatment) than in previous studies with pomalidomide
Our study is particularly relevant because RVD has become standard first-line treatment of MM, irrespective of age or transplant eligibility, making this population clinically relevant. It is important to have an effective therapy at first relapse, especially if a subsequent ASCT is considered at this stage.
Methods
Study design
This prospective, investigator-initiated, nonrandomized, multicenter, open-label, phase 2 study evaluated the efficacy and safety of salvage treatment with PCD in ASCT-eligible patients with MM in first relapse after being treated in the IFM 2009/DFCI trial.16 The IFM 2009/DFCI trial included an induction phase with RVD, followed by delayed (arm A) or upfront (arm B) ASCT, subsequent consolidation with RVD, and lenalidomide maintenance therapy for 1 year.
In this relapse study, 2 groups of patients were included. Those in group A consisted of patients who had relapsed after inclusion in arm A of the IFM/DFCI trial, and those in group B consisted of patients who had relapsed after inclusion in arm B of the IFM/DFCI trial. Group A patients underwent ASCT after 4 cycles of PCD and then received 2 cycles of PCD consolidation followed by pomalidomide-dexamethasone until disease progression. Group B patients were treated with 9 cycles of PCD, followed by pomalidomide-dexamethasone until disease progression. If the disease was not progressive after 4 cycles of salvage PCD, patients in group B could undergo a second ASCT and were then treated similarly to those in group A.
The study was approved by the medical ethics committee of each participating center in accordance with the Declaration of Helsinki. All participants provided written informed consent before inclusion. The trial was registered at www.clinicaltrials.gov as #NCT02244125.
Study population
Patients were included if they had initially been treated in the IFM 2009/DFCI trial16 and were in first relapse. A total of 100 patients (50 from the initial arm A and 50 from the upfront ASCT arm B) were enrolled between April 2014 and February 2017. The trial was conducted in 30 hospitals in France. All patients had progressive MM (symptomatic or not) and were required to have measurable disease, defined by conventional criteria as any of the following: serum monoclonal protein higher than 10 g/L, urine M-protein higher than 200 mg/24 hours, or serum immunoglobulin free light-chain higher than 100 mg/L, and abnormal serum immunoglobulin κ to λ free light-chain ratio. A World Health Organization performance status of 0 to 2, platelet count of at least 75 × 109/L, absolute neutrophil count of at least 1.0 × 109/L, hemoglobin at least 8.5 g/dL, and serum hepatic aminotransferase and bilirubin levels less than 3-fold the upper limit of normal were also required. Patients were required to have an estimated creatinine clearance of more than 50 mL/min (Cockcroft-Gault calculation) and had to agree to use contraception if conception was possible. Exclusion criteria included clinically relevant active comorbid medical or psychiatric conditions or a history of other malignancy within the last 5 years.
Drug administration
All drugs were administered orally. Pomalidomide was started at a dose of 4 mg/day for 21 days in 28-day cycles, cyclophosphamide at 300 mg/week, and dexamethasone at 40 mg/day on days 1 to 4 and days 15 to 18 for the first 4 PCD cycles, and on days 1, 8, 15, and 22 for subsequent cycles.
After the 4 salvage PCD cycles, patients in group A underwent ASCT after treatment with melphalan 200 mg/m2, followed by 2 cycles of PCD consolidation (resumed 3 months after the transplant) and then maintenance with pomalidomide-dexamethasone until disease progression. In the maintenance phase, pomalidomide was given orally at 4 mg/day on 21 days per 28-day cycle, and dexamethasone at 20 mg/day on days 1, 8, 15, and 22 per 28-day cycle.
Patients in group B received 5 additional PCD cycles and then pomalidomide-dexamethasone maintenance therapy. In the maintenance phase, pomalidomide was unchanged, similar to group A.
Antithrombotic prophylaxis comprised low-dose aspirin or low-molecular-weight heparin according to the thrombotic risk. Unless contraindicated, all patients received prophylactic anti-infective therapy with trimethoprim-sulfamethoxazole (Bactrim Forte, recommended dose: 800 mg 3 times a week, with lederfolin 15 mg 3 times a week) plus valacyclovir (Zelitrex, recommended dose: 500 mg twice daily) and amoxicillin (recommended dose: 1 g twice daily) throughout the treatment period.
Dose modifications and reasons for early cessation of trial therapy
See supplemental Data, available on the Blood Web site.
Primary and secondary objectives
The primary objective was to evaluate the rate of PR or better after 4 cycles of PCD in patients previously treated with RVD, with or without upfront ASCT. A comparison of groups A and B was planned.
The secondary objectives were to evaluate the time to response, duration of response, and safety of PCD, and to assess PFS and OS. Time to response was defined as the time from the date of inclusion to the date of the first observation of response (PR or better), and duration of response was defined as the time from the first response (PR or better) to disease progression. PFS was calculated from the start of PCD treatment until the first evidence of disease progression or date of last follow-up evaluation. OS was calculated from the beginning of PCD treatment until death from any cause or date of last contact.
Efficacy and safety assessments
Treatment response was assessed at the end of each cycle, according to the International Myeloma Working Group Uniform Response Criteria.17 If, in this period, any patient had received a different treatment, it was considered as a response failure. Response was also evaluated separately in patients with high-risk cytogenetics, as defined by the presence of t(4;14), t(14;16), del(17p), determined by fluorescence in situ hybridization on purified myeloma cells before the start of PCD treatment.
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).18 AEs of grade 1 or higher were assessed during each cycle. Safety assessments were done throughout the study, from inclusion until 30 days after administration of the last dose of study drug.
Sample size
A single-stage phase 2 study design proposed by Fleming19 was used to test the null hypothesis of the overall response rate (PR or better) at the end of the 4th cycle, with at least 50% vs the alternative hypothesis of at least 65% considered as sufficiently promising in terms of response to PCD. A sample size of 93 evaluable patients was planned for a probability of 1-sided type I error fixed at 0.05 and a power of 0.90, using A’Hern’s tables for exact single-stage phase 2 designs.20 If 55 or more PRs (or better) were observed, the null hypothesis would be rejected. By expecting a 7% loss to follow-up before PR evaluation, 100 patients were included in the study. To give the same weight to both groups in the overall evaluation, 50 patients from arm A and 50 patients from arm B were included. The intention-to-treat (ITT) population was defined as the selected population (n = 104) excluding the screen failures (n = 4), and the modified ITT (mITT) population was defined as all patients included and evaluated for the primary end point.
Statistical analysis
The results are presented as median values (1st and 3rd quartiles). The PR (or better) rate after the 4th cycle induction stage was calculated with 95% 1-sided confidence intervals (CIs), using Clopper-Pearson’s exact method on the mITT population. Univariate and multivariate analysis of predictive factors for response to PCD (age, sex, myeloma isotype, International Staging System, response to initial treatment according to the IFM 2009 trial, cytogenetic risk) was performed using Fisher’s exact test and exact logistic regression, respectively. OS and PFS survival curves were calculated using the Kaplan-Meier method. Data were analyzed using SAS software (SAS Institute, Version 9.4).
Data sharing statement
All the data are available from the corresponding author.
Results
Patient characteristics
Seven hundred patients were included in the IFM 2009/DFCI trial.16 At the beginning of enrolment in this PCD salvage study, 200 of these patients had already relapsed. A total of 134 patients who had relapsed were not included in the PCD trial.
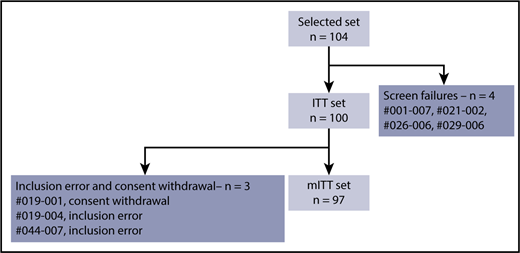
Between April 2014 and February 2017, 104 relapsed patients were enrolled at 30 IFM centers. There were 4 screening failures, 2 inclusion errors (monoclonal peak not evaluable at screening), and 1 consent withdrawal (patient withdrew during the first cycle). Thus, the mITT population consisted of 97 patients (Figure 1).
The cutoff date for the final analysis of the primary end point was October 25, 2017. The characteristics of the patients at inclusion in the PCD trial are summarized in Table 1. Median time from MM diagnosis to PCD treatment was 3.6 years (3.1–4.2 years, 1st and 3rd quartile), and the median time from stopping lenalidomide maintenance and inclusion in the PCD trial was 22.6 months (16.7-29.5 months, 1st and 3rd quartile). No patients were progressive on lenalidomide maintenance or refractory to lenalidomide. Ninety-seven percent of patients had a World Health Organization performance status of 0 or 1. International Staging System was I (67%), II (12%), or III (6%). Five patients had a plasmacytoma and 12% had high-risk cytogenetics (t[4;14] and/or del 17p and/or t[14;16]). There was no difference in patient characteristics between group A and group B except for the time from inclusion in the IFM/DFCI trial to the time of inclusion in the relapse trial, which was longer for patients in group B (median, 3.6 years; 3-4.5 quartile) than in group A (median, 3.4 years; 2.8-3.8 quartile; P = .0076).
Baseline characteristics of the study population (n = 100)
| Characteristics . | . |
|---|---|
| Male | 62% |
| Age, y, median (IQR) | 62 (39–70) |
| Initial therapy: RVD alone/RVD + ASCT | 50%/50% |
| Time from diagnosis to PCD treatment, y, median (IQR) | 3.6 (3.1-4.2) |
| Time from stopping lenalidomide maintenance to PCD treatment, mo, median (IQR) | 22.6 (16.7-29.5) |
| World Health Organization performance status (0 or 1) | 97% |
| Type of myeloma | |
| Immunoglobulin G | 73% |
| Immunoglobulin A | 15% |
| Light chain | 10% |
| Other | 2% |
| International Staging System | |
| I | 67% |
| II | 12% |
| III | 6% |
| Unknown | 15% |
| Serum lactate dehydrogenase | |
| Normal | 77% |
| Elevated | 21% |
| Unknown | 2% |
| Cytogenetic abnormalities | |
| Standard risk | 69% |
| High risk: del 17p or t(14;16) or t(14;16) | 12% |
| Unknown | 19% |
| Plasmacytoma | 5% |
| Characteristics . | . |
|---|---|
| Male | 62% |
| Age, y, median (IQR) | 62 (39–70) |
| Initial therapy: RVD alone/RVD + ASCT | 50%/50% |
| Time from diagnosis to PCD treatment, y, median (IQR) | 3.6 (3.1-4.2) |
| Time from stopping lenalidomide maintenance to PCD treatment, mo, median (IQR) | 22.6 (16.7-29.5) |
| World Health Organization performance status (0 or 1) | 97% |
| Type of myeloma | |
| Immunoglobulin G | 73% |
| Immunoglobulin A | 15% |
| Light chain | 10% |
| Other | 2% |
| International Staging System | |
| I | 67% |
| II | 12% |
| III | 6% |
| Unknown | 15% |
| Serum lactate dehydrogenase | |
| Normal | 77% |
| Elevated | 21% |
| Unknown | 2% |
| Cytogenetic abnormalities | |
| Standard risk | 69% |
| High risk: del 17p or t(14;16) or t(14;16) | 12% |
| Unknown | 19% |
| Plasmacytoma | 5% |
Efficacy of PCD
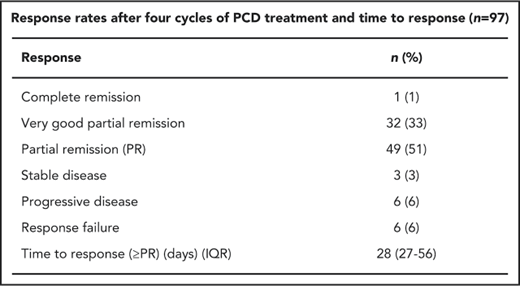
Ninety-seven patients were evaluated. There were 6 response failures (3 in each arm) related to treatment discontinuation before the end of cycle 4. After 4 cycles of PCD, objective responses were obtained in 82 patients (85%): complete remission (n = 1; 1%), very good PR (VGPR; n = 32, 33%), and PR (n = 49, 51%). Stable disease was observed in 3 patients (3%), and progressive disease in 6 (6%). PR or better was observed in 82/97 patients (85%), and VGPR or better was observed in 33/97 patients (34%). Median time to response (≥PR) was 28 days (27-56 interquartile range; Table 2). Similar results were observed in both study arms (Table 3). PR (or better) rate in group A and group B was 85% and 84%, and VGPR (or better) rate was 35% and 33% (Table 3). Median duration of response was 33.5 months (95% CI, 26.3 not evaluable [NE] months). By univariate analysis, sex, age, type of immunoglobulin, International Staging System score, cytogenetics, and group A or group B did not influence the response (PR or better). Exact logistic regression confirmed these results (supplemental Data). However, patients who reached at least VGPR with initial first-line treatment according to the IFM 2009 protocol were less at risk of not responding to PCD compared with those who did not reach VGPR (6.7% vs 25%; P = .048). There were too many missing cytogenetic data for any valuable analysis.
Response rates after 4 cycles of PCD treatment and time to response (n = 97)
| Response . | n (%) . |
|---|---|
| Complete remission | 1 (1) |
| Very good PR | 32 (33) |
| PR | 49 (51) |
| Stable disease | 3 (3) |
| Progressive disease | 6 (6) |
| Response failure | 6 (6) |
| Time to response (≥PR), d (IQR) | 28 (27-56) |
| Response . | n (%) . |
|---|---|
| Complete remission | 1 (1) |
| Very good PR | 32 (33) |
| PR | 49 (51) |
| Stable disease | 3 (3) |
| Progressive disease | 6 (6) |
| Response failure | 6 (6) |
| Time to response (≥PR), d (IQR) | 28 (27-56) |
Values shown are n (%), unless stated otherwise.
Responses were assessed according to the International Uniform Response Criteria for Multiple Myeloma.
Response rates after 4 cycles of PCD treatment and time to response in group A versus group B
| Response . | Group A(n = 48) . | Group B(n = 49) . | P . |
|---|---|---|---|
| Complete remission | 1 (2) | 0 (0) | |
| Very good PR | 16 (33) | 16 (33) | |
| PR | 24 (50) | 25 (51) | |
| Stable disease | 2 (4) | 1 (2) | |
| Progressive disease | 2 (4) | 4 (8) | |
| Response failure | 3 (6) | 3 (6) | |
| Partial remission or better | 41 (85) | 41 (84) | 1 |
| Time to response (≥PR), d (IQR) | 28 (27-56) | 28(27-55) | .71 |
| Response . | Group A(n = 48) . | Group B(n = 49) . | P . |
|---|---|---|---|
| Complete remission | 1 (2) | 0 (0) | |
| Very good PR | 16 (33) | 16 (33) | |
| PR | 24 (50) | 25 (51) | |
| Stable disease | 2 (4) | 1 (2) | |
| Progressive disease | 2 (4) | 4 (8) | |
| Response failure | 3 (6) | 3 (6) | |
| Partial remission or better | 41 (85) | 41 (84) | 1 |
| Time to response (≥PR), d (IQR) | 28 (27-56) | 28(27-55) | .71 |
Values shown are n (%), unless stated otherwise. Group A: relapsing patients without previous ASCT (from arm A of IFM 2009 trial), Group B: relapsing patients with a previous ASCT (from arm B of IFM 2009 trial).
Forty-eight patients in group A had not previously received an ASCT and were scheduled to receive an ASCT at first relapse. Among these 48 patients, 45 (94%) could proceed to ASCT. Seven of the 49 patients in group B received a second ASCT.
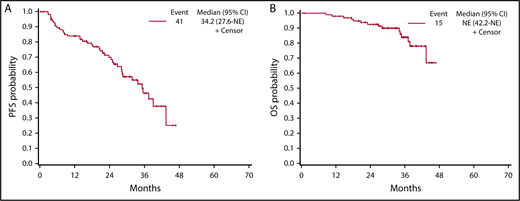
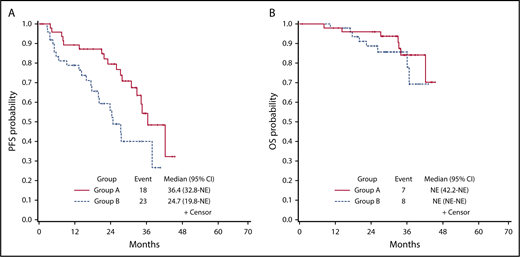
After a median follow-up of 33.6 months (interquartile range [IQR], 28.0-37.5 months), 14 patients had died because of disease progression, and 1 as a result of colorectal cancer. A total of 41 events occurred: 34/100 patients had disease progression and 7/15 died without progression. As shown in Figure 2, PFS was estimated at 84.1% (95% CI, 77.0%-91.8%), 68.4% (95% CI, 59.1%-79.3%), and 46.4% (95% CI, 34.8%-61.8%) at 12, 24, and 36 months from the beginning of PCD treatment, respectively (Figure 2). At the same points, OS was estimated at 98% (95% CI, 95.2%-100%), 92.6% (95% CI, 87.5%-98.0%), and 84.1% (95% CI, 75.7%-93.3%), respectively (Figure 2). PFS and OS in group A and group B are shown Figure 3A-B. In group A, PFS was estimated at 89.4% (95% CI, 81.0%-98.6%), 79.6% (95% CI, 68.4%-92.5%), and 54.5% (95% CI, 39.2%-75.8%), and OS was estimated at 98.0% (95% CI, 94.1%-100%), 95.9% (95% CI, 90.5%-100%), and 84.2% (95% CI, 73.1%-97.0%), at 12, 24, and 36 months from the beginning of PCD treatment, respectively. In group B, PFS was estimated at 78.8% (95% CI, 68.0%-91.4%), 55.9% (95% CI, 42.0%-74.3%), and 40.0% (95% CI, 25.8%-62.1%), and OS was estimated at 98.0% (95% CI, 94.2%-100%), 88.7% (95% CI, 79.9%-98.6%), and 85.8% (95% CI, 75.7%-97.2%), at 12, 24, and 36 months from the beginning of PCD treatment, respectively.
Kaplan-Meier curves. PFS in group A vs B (A), and OS in group A vs B (B).
Tolerability
After 4 cycles of PCD, grade 3 to 4 AEs occurred in 73/100 patients (73%). These included 62% hematological toxicities (mainly neutropenia [51%] and lymphopenia [37%]), 9% infections (67% pneumonia), 2% asthenia, 3% hyperglycemia, 3% gastrointestinal disorders, 1% allergic skin reactions, and 3% cardiovascular disorders (1 pulmonary embolism, 1 myocardial infarction, and 1 syncope). During the first 4 cycles of PCD, 17% of patients at some point received granulocyte colony-stimulating factor support. AEs occurring in at least 3% of patients are shown in Table 4. There was no grade 3 or 4 peripheral neuropathy. Six patients (6%) discontinued pomalidomide, 8% cyclophosphamide, and 9% dexamethasone. Dose reductions were recorded in 34% of patients for pomalidomide, 29% for cyclophosphamide, and 40% for dexamethasone, the reasons being an AE/serious AE (77%, 71%, and 71%, respectively), or “other” (23%, 29%, and 29%, respectively).
Adverse events occurring in at least 3% of patients after 4 cycles of PCD (n = 100)
| . | Grade 1 to 2, n (%) . | Grade 3 to 4, n (%) . |
|---|---|---|
| Adverse event | 97 (97%) | 73 (73%) |
| Blood and lymphatic system disorders | 25 (25%) | 100 (100%) |
| Anemia | 9 (9%) | 7 (7%) |
| Neutropenia | 8 (8%) | 51 (51%) |
| Lymphopenia | 4 (4%) | 37 (37%) |
| Thrombocytopenia | 4 (4%) | 5 (5%) |
| Gastrointestinal disorders | 71 (71%) | 2 (2%) |
| Constipation | 23 (23%) | 1 (1%) |
| Diarrhea | 16 (16%) | 0 (0%) |
| Nausea | 12 (12%) | 0 (0%) |
| Abdominal pain (upper) | 11 (11%) | 1 (1%) |
| Vomiting | 5 (5%) | 0 (0%) |
| Hemorrhoids | 4 (4%) | 0 (0%) |
| Constitutional | 65 (65%) | 3 (3%) |
| Asthenia | 50 (50%) | 3 (3%) |
| Edema (peripheral) | 15 (15%) | 0 (0%) |
| Immune system disorders | 8 (8%) | 3 (3%) |
| Hypersensitivity | 8 (8%) | 3 (3%) |
| Infections | 49 (49%) | 6 (6%) |
| Bronchitis | 25 (25%) | 0 (0%) |
| Nasopharyngitis | 9 (9%) | 0 (0%) |
| Gastroenteritis | 8 (8%) | 0 (0%) |
| Sinusitis | 4 (4%) | 0 (0%) |
| Pneumonia | 3 (3%) | 6 (6%) |
| Metabolism | 7 (7%) | 4 (3%) |
| Hyperglycemia | 4 (4%) | 4 (3%) |
| Hypokalemia | 3 (3%) | 0 (0%) |
| Musculoskeletal | 51 (51%) | 2 (2%) |
| Muscle spasms | 22 (22%) | 0 (0%) |
| Bone pain | 20 (20%) | 2 (2%) |
| Muscular weakness | 5 (5%) | 0 (0%) |
| Myalgia | 4 (4%) | 0 (0%) |
| Nervous system disorders | 25 (25%) | 0 (0%) |
| Tremor | 14 (14%) | 0 (0%) |
| Peripheral sensory neuropathy | 7 (7%) | 0 (0%) |
| Dysesthesia | 4 (4%) | 0 (0%) |
| Psychiatric disorders | 47 (47%) | 1 (1%) |
| Insomnia | 22 (22%) | 1 (1%) |
| Irritability | 7 (7%) | 0 (0%) |
| Sleep disorder | 6 (6%) | 0 (0%) |
| Agitation | 4 (4%) | 0 (0%) |
| Anxiety | 4 (4%) | 0 (0%) |
| Depression | 4 (4%) | 0 (0%) |
| Respiratory, thoracic and mediastinal disorders | 39 (39%) | 3 (3%) |
| Dyspnea | 15 (15%) | 2 (2%) |
| Cough | 11 (11%) | 1 (1%) |
| Dysphonia | 5 (5%) | 0 (0%) |
| Dyspnea exertional | 4 (4%) | 0 (0%) |
| Hiccups | 4 (4%) | 0 (0%) |
| Skin disorders | 25 (25%) | 1 (1%) |
| Rash | 10 (10%) | 1 (1%) |
| Erythema | 9 (9%) | 0 (0%) |
| Hyperhidrosis | 6 (6%) | 0 (0%) |
| Vascular disorders | 5 (5%) | 0 (0%) |
| Flushing | 5 (5%) | 0 (0%) |
| . | Grade 1 to 2, n (%) . | Grade 3 to 4, n (%) . |
|---|---|---|
| Adverse event | 97 (97%) | 73 (73%) |
| Blood and lymphatic system disorders | 25 (25%) | 100 (100%) |
| Anemia | 9 (9%) | 7 (7%) |
| Neutropenia | 8 (8%) | 51 (51%) |
| Lymphopenia | 4 (4%) | 37 (37%) |
| Thrombocytopenia | 4 (4%) | 5 (5%) |
| Gastrointestinal disorders | 71 (71%) | 2 (2%) |
| Constipation | 23 (23%) | 1 (1%) |
| Diarrhea | 16 (16%) | 0 (0%) |
| Nausea | 12 (12%) | 0 (0%) |
| Abdominal pain (upper) | 11 (11%) | 1 (1%) |
| Vomiting | 5 (5%) | 0 (0%) |
| Hemorrhoids | 4 (4%) | 0 (0%) |
| Constitutional | 65 (65%) | 3 (3%) |
| Asthenia | 50 (50%) | 3 (3%) |
| Edema (peripheral) | 15 (15%) | 0 (0%) |
| Immune system disorders | 8 (8%) | 3 (3%) |
| Hypersensitivity | 8 (8%) | 3 (3%) |
| Infections | 49 (49%) | 6 (6%) |
| Bronchitis | 25 (25%) | 0 (0%) |
| Nasopharyngitis | 9 (9%) | 0 (0%) |
| Gastroenteritis | 8 (8%) | 0 (0%) |
| Sinusitis | 4 (4%) | 0 (0%) |
| Pneumonia | 3 (3%) | 6 (6%) |
| Metabolism | 7 (7%) | 4 (3%) |
| Hyperglycemia | 4 (4%) | 4 (3%) |
| Hypokalemia | 3 (3%) | 0 (0%) |
| Musculoskeletal | 51 (51%) | 2 (2%) |
| Muscle spasms | 22 (22%) | 0 (0%) |
| Bone pain | 20 (20%) | 2 (2%) |
| Muscular weakness | 5 (5%) | 0 (0%) |
| Myalgia | 4 (4%) | 0 (0%) |
| Nervous system disorders | 25 (25%) | 0 (0%) |
| Tremor | 14 (14%) | 0 (0%) |
| Peripheral sensory neuropathy | 7 (7%) | 0 (0%) |
| Dysesthesia | 4 (4%) | 0 (0%) |
| Psychiatric disorders | 47 (47%) | 1 (1%) |
| Insomnia | 22 (22%) | 1 (1%) |
| Irritability | 7 (7%) | 0 (0%) |
| Sleep disorder | 6 (6%) | 0 (0%) |
| Agitation | 4 (4%) | 0 (0%) |
| Anxiety | 4 (4%) | 0 (0%) |
| Depression | 4 (4%) | 0 (0%) |
| Respiratory, thoracic and mediastinal disorders | 39 (39%) | 3 (3%) |
| Dyspnea | 15 (15%) | 2 (2%) |
| Cough | 11 (11%) | 1 (1%) |
| Dysphonia | 5 (5%) | 0 (0%) |
| Dyspnea exertional | 4 (4%) | 0 (0%) |
| Hiccups | 4 (4%) | 0 (0%) |
| Skin disorders | 25 (25%) | 1 (1%) |
| Rash | 10 (10%) | 1 (1%) |
| Erythema | 9 (9%) | 0 (0%) |
| Hyperhidrosis | 6 (6%) | 0 (0%) |
| Vascular disorders | 5 (5%) | 0 (0%) |
| Flushing | 5 (5%) | 0 (0%) |
Maintenance treatment with pomalidomide-dexamethasone could be initiated in 75% of enrolled patients, and median duration of maintenance was 16.6 months in group A vs 11.9 months in group B. Steroids were stopped in 16% (12/74) of patients, mainly because of AEs, whereas only 5% (4/74) patients stopped pomalidomide.
Two patients experienced a second primary malignancy: 1 baso-cellular carcinoma and 1 colon adenocarcinoma.
Discussion
PCD was associated with a PR (or better) rate of 85%, and prolonged PFS and OS in ASCT-eligible MM patients who had relapsed after first-line treatment with RVD, regardless of whether they received an ASCT upfront or not. It has been reported that the addition of cyclophosphamide to an immunomodulatory drug could increase the efficacy of the latter, and even rescue lenalidomide-refractory patients.21 Similarly, the addition of cyclophosphamide to pomalidomide-dexamethasone in lenalidomide-refractory MM increased the overall response rate from 39% to 65%, and median PFS from 4.4 to 9.5 months.15 Larocca et al showed that pomalidomide in combination with cyclophosphamide-prednisone is effective and well-tolerated in lenalidomide- and bortezomib-refractory patients with MM (≥PR: 50%; median PFS: 8.6 months).14 Our findings in ASCT-eligible patients with MM in first relapse confirm these results with a PR (or better) rate of 85% and a VGPR (or better) rate of 34% after 4 cycles of PCD.
RVD has become the foundation first-line treatment of MM in transplant eligible and noneligible patients.22 We show that PCD can rescue these patients after relapse, with more than 90% of patients who did not receive an upfront ASCT proceeding to ASCT. This is now our first choice of sequential treatment in the relapse setting. It is important to plan the management of relapses when considering first-line treatment of MM.23
Pomalidomide may be effective in patients with high-risk cytogenetics, especially those harboring the 17p deletion and, to a lesser extent, the (4;14) translocation.24 We were unable to carry out a statistical analysis of this population because of the high number of missing data. Pomalidomide has also been reported to be safe in patients with renal failure, but this was an exclusion criterion in our study.
The efficacy of pomalidomide plus oral cyclophosphamide in lenalidomide-exposed MM raises the question of the mechanisms of action of this regimen. Low-dose cyclophosphamide has multiple effects, including direct antitumor activity, antiangiogenic effects,25,26 microenvironment modulation,27 and improvement of T-cell and natural killer cell-mediated antitumor immune responses via depletion of regulatory T cells.28 Pomalidomide has a dual mechanism of action, having direct antitumor as well as immunomodulatory effects that include the activation and proliferation of many immune cells including dendritic cells, T cells, natural killer T cells, and natural killer cells. This dual mechanism of action may in part explain the synergy between these drugs in lenalidomide-exposed patients.29
AEs in our patients were manageable and were in line with previous experience with pomalidomide. When cyclophosphamide was added to pomalidomide-prednisone, hematologic toxicity was comparable to that observed with pomalidomide-dexamethasone.30,31 Toxicity was mostly hematologic, and one-third of patients experienced a decrease in dose of either pomalidomide or cyclophosphamide. Compared with a previous study,15 cyclophosphamide was given at 300 mg/week instead of 400 mg/week, and every week instead of 3 times/mo to decrease hematotoxicity. Cytopenia was still an issue with concurrent infections. Decreasing pomalidomide to 3 mg instead of 4 mg with the same schedule from day 1 to day 21 every 28 days may be an alternative. Altogether, this suggests that low-dose cyclophosphamide does not significantly increase hematologic toxicity when added to pomalidomide. In contrast, melphalan causes more profound myelosuppression, making this alkylating agent less attractive to use in combination with an immunomodulatory drug.32 Nonhematologic toxicity of PCD consisted mainly of infections. Two cases of second malignancy have been reported to date, but the follow-up time is too short to be conclusive.
By design, this study addressed a selected population. When enrolment started, 200/700 patients included in the IFM 2009 trial had already relapsed. No RVD-refractory patient was included in this study. Moreover, because only 30 IFM centers participated, many patients relapsing after IFM 2009 were not included. This may have resulted in patient selection bias.
A PFS/OS comparison of the 2 groups was not planned in this study because these 2 groups differed in nature. Patients in arm A had not received an ASCT, and their first relapse was earlier than in arm B. However, the other characteristics of the 2 groups were similar at inclusion, as well as the responses after 4 PCD cycles. For these reasons, we reported PFS and OS within each group without comparing them.
Many trials have added a third drug to pomalidomide-dexamethasone, but always in patients who had received many lines of treatment and/or who were refractory to lenalidomide-bortezomib. Proteasome inhibitors, such as bortezomib, in combination with pomalidomide and dexamethasone, either as retreatment or in relapse, have been evaluated, as well as more recent proteasome inhibitors such as carfilzomib and ixazomib.33-36 However, intertrial comparisons must be interpreted with caution because they may be biased by differences in patient populations and study design. Nevertheless, this combination may be an ideal backbone for future studies in conjunction with monoclonal antibodies such as the anti-SLAMF737 or the anti-CD38 monoclonal antibodies, daratumumab or isatuximab,38,39 given its favorable safety profile and oral administration.
Although several new agents to treat lenalidomide- and bortezomib-refractory MM have been approved by the US Food and Drug Administration and/or European Medicines Agency, many of these studies had low numbers or, by design, excluded patients who were exposed and/or refractory to lenalidomide. In addition, these therapies are not yet available or reimbursed in many countries, whereas cyclophosphamide is widely available. PCD is a fully oral, triplet combination, which is convenient for patients and is also associated with lower costs of patient care. This highlights the importance of this effective first relapse strategy for relapsed patients with MM previously exposed to bortezomib-lenalidomide early in the course of their disease.
In summary, fully oral, relatively cost-effective PCD was highly effective and safe as second-line treatment in RVD-exposed patients. Addition of a monoclonal antibody could increase its efficacy further.
Presented in abstract form at the the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the data and safety monitoring committee (Michel Delforge, Jean-Yves Mary), and representatives of the Curie Institute and Intergroupe Francophone du Myélome, Paris, who were involved in the data collection and analyses (Christine Foulon and Marie Odile Petillon).
This work was supported by the Curie Institute. Celgene supplied pomalidomide free of charge; however, Celgene was not involved in the study design, collection, analysis or interpretation of data, or the writing of the report.
Authorship
Contribution: L.G., F.K., B.B., C.M., and M.A. designed the study; L.G., F.K., M.R., M.E.-B., I.L., T.F., X.L., L.K., A.P., P.M., G.M., A.-M.S., B.R., C.C., M.T., C.A., P.L., M.M., E.V., L.B., O.A., E.J., F.O.-P., S.B., J.-R.E., K.B., M.W., B.P., A.J., J.-C.E., S.G., M.M., and C.H. enrolled patients; L.G., F.K., B.B., H.A.L., C.M., and M.A. analyzed the data; and L.G., F.K., B.B., C.M., and M.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurent Garderet, Hôpital Saint Antoine, Service d’Hématologie et Thérapie Cellulaire, 184, rue du Faubourg Saint Antoine, 75012, Paris, France; e-mail: laurent.garderet@aphp.fr.
REFERENCES
Author notes
L.G. and F.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal