Abstract
Galectins and cytokines are both secreted proteins whose levels are prognosis factors for several cancers. Extracellular galectins bind to the glycans decorating glycoproteins and are overproduced in most cancers. Accumulative evidence shows that galectins regulate cytokines during cancer progression. Although galectins alter cytokine function by binding to the glycans decorating cytokines or their receptors, cytokines could also regulate galectin expression and function. This review revises these complex interactions and their clinical impact, particularly in hematological cancers.
Introduction
Galectins are a family of 17 proteins that share a common carbohydrate-binding domain. They are ubiquitous proteins found in the nucleus, cytoplasm, and the extracellular space. Intracellular galectins presumably establish protein-protein interactions, whereas extracellular galectins interact mainly with glycans decorating glycoproteins.1,2 The main sources of extracellular galectins in cancer are myeloid and tumor cells, but other cells such as mesenchymal cells and lymphoid cells can also secrete galectins.3-6 All galectins are multivalent because of either oligomerization or their multiple carbohydrate-binding domains per protein.7 Galectins bind glycans in a loosely specific and highly cooperative fashion: they recognize several glycan patterns and form galectin-glycan lattices whose binding strength correlates with the number of interactions established. Galectins are involved in a wide range of processes in both homeostasis and disease. Some galectins are overproduced in human malignancies and autoimmune diseases.3,4 High galectin expression is often an independent unfavorable prognostic factor for disease progression in different cancers (see Table 1 for a complete list regarding hematological malignancies).8
Hematological malignancies where galectins are upregulated and associated with clinical features
| Hematological malignancy . | Galectin . | Function/associated with . | Reference . | |
|---|---|---|---|---|
| Type . | Subtype . | |||
| Lymphoma | Hodgkin | Galectin-1 | Tumor burden | 55 |
| Adverse clinical features | ||||
| Non-Hodgkin | Galectin-1 (ALCL) | Invasion | 56 | |
| Galectin-1 (CTCL) | Immune evasion (Th2 tolerogenic environment) | 57 | ||
| Galectin-3 (DLBCL, PEL) | Protection from Fas-induced apoptosis | 58 | ||
| Galectin-7 | Increase metastasis | 59 | ||
| Low survival (mouse model) | ||||
| Leukemia | PCL | Galectin-1 | Aggressiveness | 18 |
| Pre–B-ALL | Galectin-3 | Drug resistance | 60 | |
| AML | Galectin-3 | High primary refractory rate | 61 | |
| Low overall survival | 62,63 | |||
| Drug resistance | 34 | |||
| Galectin-9 | Immune evasion | 39,40 | ||
| Galectin-12 | Low overall survival | 64 | ||
| CLL | Galectin-1 | Protumoral stroma | 17 | |
| Galectin-9 | Progression of the disease | 65 | ||
| CML | Galectin-1 | Chemo-resistance | 66 | |
| Galectin-3 | Drug resistance | 35 | ||
| APL | Galectin-3 | Low relapse-free survival | 62 | |
| MM | Galectin-1 | Aggressiveness | 18 | |
| Low overall survival | ||||
| Galectin-3 | Protumoral | 44 | ||
| Galectin-9 | Antiproliferative | 67 | ||
| Hematological malignancy . | Galectin . | Function/associated with . | Reference . | |
|---|---|---|---|---|
| Type . | Subtype . | |||
| Lymphoma | Hodgkin | Galectin-1 | Tumor burden | 55 |
| Adverse clinical features | ||||
| Non-Hodgkin | Galectin-1 (ALCL) | Invasion | 56 | |
| Galectin-1 (CTCL) | Immune evasion (Th2 tolerogenic environment) | 57 | ||
| Galectin-3 (DLBCL, PEL) | Protection from Fas-induced apoptosis | 58 | ||
| Galectin-7 | Increase metastasis | 59 | ||
| Low survival (mouse model) | ||||
| Leukemia | PCL | Galectin-1 | Aggressiveness | 18 |
| Pre–B-ALL | Galectin-3 | Drug resistance | 60 | |
| AML | Galectin-3 | High primary refractory rate | 61 | |
| Low overall survival | 62,63 | |||
| Drug resistance | 34 | |||
| Galectin-9 | Immune evasion | 39,40 | ||
| Galectin-12 | Low overall survival | 64 | ||
| CLL | Galectin-1 | Protumoral stroma | 17 | |
| Galectin-9 | Progression of the disease | 65 | ||
| CML | Galectin-1 | Chemo-resistance | 66 | |
| Galectin-3 | Drug resistance | 35 | ||
| APL | Galectin-3 | Low relapse-free survival | 62 | |
| MM | Galectin-1 | Aggressiveness | 18 | |
| Low overall survival | ||||
| Galectin-3 | Protumoral | 44 | ||
| Galectin-9 | Antiproliferative | 67 | ||
ALCL, anaplastic large-cell lymphoma; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; CLL, chronic lymphoid leukemia; CML, chronic myeloid leukemia; CTCL, cutaneous T-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; PCL, plasma cell leukemia; PEL, pure erythroid leukemia; pre–B-ALL, acute lymphoblastic leukemia.
Cytokines are intercellular signals secreted by different cell types as soluble small proteins. They regulate cell fate, particularly immune cell fate, by interacting with specific receptors at the target cell surface and then triggering different cell programs. Many cytokines, like most secreted proteins, are posttranslationally decorated with glycans. Cytokine glycosylation is known to determine the cytokine’s biological activity and stability.9
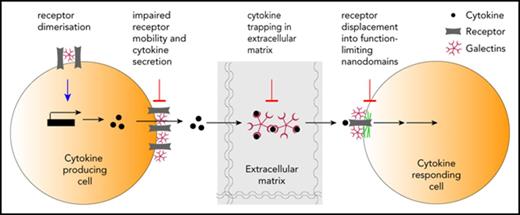
Although galectins and cytokines seem reciprocally controlled, more is known about how galectins affect cytokines.10 The currently available data on this subject represent mainly observations and correlations, with only few articles digging deeper into the regulatory mechanism. Moreover, results are often biased by the authors’ scientific focus on a specific galectin. Thus, we cannot exclude that another galectin might be having the same or an opposing effect on cytokine regulation to the galectin studied. Furthermore, many also neglect to investigate the role of extracellular vs intracellular galectins on cytokine regulation. In this article, we discuss some of the galectin-cytokine interactions that have important consequences in the progression of mostly hematological and some nonhematological cancers (a thorough list is shown in Table 2). Of particular interest are the emerging ways in which extracellular galectins regulate cytokines by affecting their levels of expression and secretion, by hampering the diffusion of cytokines through the extracellular matrix, and/or by modulating cytokine signaling through its corresponding receptor (Figure 1). We integrate the available data for galectins, propose some hypotheses, and raise important questions in the field.
Galectins and cytokines reciprocal regulation
| Galectin . | Effect on cytokines . | Source of cytokine . | Clinical consequence . | Reference . |
|---|---|---|---|---|
| Galectin-1 | ↑ IL-10 | T cells | In vitro data | 12 |
| CLL | Low survival | 17 | ||
| DCs | Protective for autoimmune mouse model (EAE) | 11 | ||
| ↑ IL-5 | T cells | Protective for autoimmune mouse model (RA) | 13 | |
| ↑ CCL3 | CLL | Low overall survival | 17 | |
| ↑ CCL2 | MM | High tumor growth and angiogenesis | 18 | |
| ↑ MMP9 | MM | High tumor growth and angiogenesis | 18 | |
| ↓ IFN-γ | T cells | Protective for autoimmune mouse model (RA, hepatitis, colitis) | 12,,-15 | |
| DCs | Protective for autoimmune mouse model (EAE) | 11 | ||
| ↓ IL-2 | T cells | Protective for autoimmune mouse model (RA) | 13 | |
| ↓ CXCL10 | MM | High tumor growth and angiogenesis | 18 | |
| ↓ TNF-α | T cells | Protective for autoimmune mouse model (hepatitis, colitis) | 14,15 | |
| ↓ IL-1b | — | Protective for autoimmune mouse model (colitis) | 15 | |
| ↓ IL-12 | — | Protective for autoimmune mouse model (EAE and colitis) | 11,15 | |
| ↓ IL-17 | DCs | Protective for autoimmune mouse model (EAE) | 11 | |
| Galectin-2 | ↑ IL-6 | Endothelial cells | — | 24 |
| ↑ G-CSF | ||||
| Galectin-3 | ↑ IL-6 | BMMSCs | Neuroblastoma survival, angiogenesis, and immune tolerance | 30 |
| Endothelial cells | Metastasis of colon cancer | 26 | ||
| ↑ IL-8 | PDAC | High tumor growth and metastasis | 25 | |
| ↑ CCL2 | ||||
| ↑ CXCL1 | ||||
| ↑ G-CSF | Endothelial cells | Metastasis of colon cancer | 26 | |
| ✖ IFN-γ signaling | Mendelian susceptibility to mycobacterial infections | 45 | ||
| ✖ IFN-γ diffusion | High tumor growth and lower T-cell infiltration | 46 | ||
| ✖ IL-12 diffusion | In vitro data | |||
| Galectin-4 | ↓ CCL2 | Colorectal cancer cell lines | Low colorectal tumor proliferation | 37 |
| ↓ CCL5 | ||||
| ↓ IL-6 | T cells | In vitro data | 36 | |
| ↓ IL-8 | ||||
| ↓ IL-10 | ||||
| ↓ TNF-α | ||||
| Galectin-7 | ↑ MMP9 | Lymphoma | High metastatic capacity | 59 |
| Galectin-9 | ↓ IFN-γ | T cells (AML) | Disease progression and low survival rate for a mouse model | 40* |
| ↓ TNF-α | ||||
| ↓ IL-2 | ||||
| ↑ TNF-α | Myeloid cells | Greater severity of EAE mouse model | 68 | |
| ↑ IFN-γ | NKs | In vitro data | 69 | |
| ↓ IFN-γ | NKs (AML) | in vitro data | 39 | |
| Galectin . | Effect on cytokines . | Source of cytokine . | Clinical consequence . | Reference . |
|---|---|---|---|---|
| Galectin-1 | ↑ IL-10 | T cells | In vitro data | 12 |
| CLL | Low survival | 17 | ||
| DCs | Protective for autoimmune mouse model (EAE) | 11 | ||
| ↑ IL-5 | T cells | Protective for autoimmune mouse model (RA) | 13 | |
| ↑ CCL3 | CLL | Low overall survival | 17 | |
| ↑ CCL2 | MM | High tumor growth and angiogenesis | 18 | |
| ↑ MMP9 | MM | High tumor growth and angiogenesis | 18 | |
| ↓ IFN-γ | T cells | Protective for autoimmune mouse model (RA, hepatitis, colitis) | 12,,-15 | |
| DCs | Protective for autoimmune mouse model (EAE) | 11 | ||
| ↓ IL-2 | T cells | Protective for autoimmune mouse model (RA) | 13 | |
| ↓ CXCL10 | MM | High tumor growth and angiogenesis | 18 | |
| ↓ TNF-α | T cells | Protective for autoimmune mouse model (hepatitis, colitis) | 14,15 | |
| ↓ IL-1b | — | Protective for autoimmune mouse model (colitis) | 15 | |
| ↓ IL-12 | — | Protective for autoimmune mouse model (EAE and colitis) | 11,15 | |
| ↓ IL-17 | DCs | Protective for autoimmune mouse model (EAE) | 11 | |
| Galectin-2 | ↑ IL-6 | Endothelial cells | — | 24 |
| ↑ G-CSF | ||||
| Galectin-3 | ↑ IL-6 | BMMSCs | Neuroblastoma survival, angiogenesis, and immune tolerance | 30 |
| Endothelial cells | Metastasis of colon cancer | 26 | ||
| ↑ IL-8 | PDAC | High tumor growth and metastasis | 25 | |
| ↑ CCL2 | ||||
| ↑ CXCL1 | ||||
| ↑ G-CSF | Endothelial cells | Metastasis of colon cancer | 26 | |
| ✖ IFN-γ signaling | Mendelian susceptibility to mycobacterial infections | 45 | ||
| ✖ IFN-γ diffusion | High tumor growth and lower T-cell infiltration | 46 | ||
| ✖ IL-12 diffusion | In vitro data | |||
| Galectin-4 | ↓ CCL2 | Colorectal cancer cell lines | Low colorectal tumor proliferation | 37 |
| ↓ CCL5 | ||||
| ↓ IL-6 | T cells | In vitro data | 36 | |
| ↓ IL-8 | ||||
| ↓ IL-10 | ||||
| ↓ TNF-α | ||||
| Galectin-7 | ↑ MMP9 | Lymphoma | High metastatic capacity | 59 |
| Galectin-9 | ↓ IFN-γ | T cells (AML) | Disease progression and low survival rate for a mouse model | 40* |
| ↓ TNF-α | ||||
| ↓ IL-2 | ||||
| ↑ TNF-α | Myeloid cells | Greater severity of EAE mouse model | 68 | |
| ↑ IFN-γ | NKs | In vitro data | 69 | |
| ↓ IFN-γ | NKs (AML) | in vitro data | 39 | |
| Cytokine . | Effect on galectins . | Source of galectins . | Clinical consequence . | Reference . |
|---|---|---|---|---|
| TNF-α/IFN-γ | ↑ Galectin-9 | MSC | In vitro data | 47 |
| IL-10 | ↑ Galectin-3 binding | — | CD8 T cell higher threshold for activation | 50 |
| Cytokine . | Effect on galectins . | Source of galectins . | Clinical consequence . | Reference . |
|---|---|---|---|---|
| TNF-α/IFN-γ | ↑ Galectin-9 | MSC | In vitro data | 47 |
| IL-10 | ↑ Galectin-3 binding | — | CD8 T cell higher threshold for activation | 50 |
BMMSC, bone marrow mesenchymal stem cells; EAE, experimental autoimmune encephalomyelitis; NK, natural killer, PDAC, pancreatic ductal adenocarcinoma; RA, rheumatoid arthritis. ↑, upregulation; ↓, downregulation; ✖, blocking.
This effect might be Tim-3 mediated and galectin-9 independent.
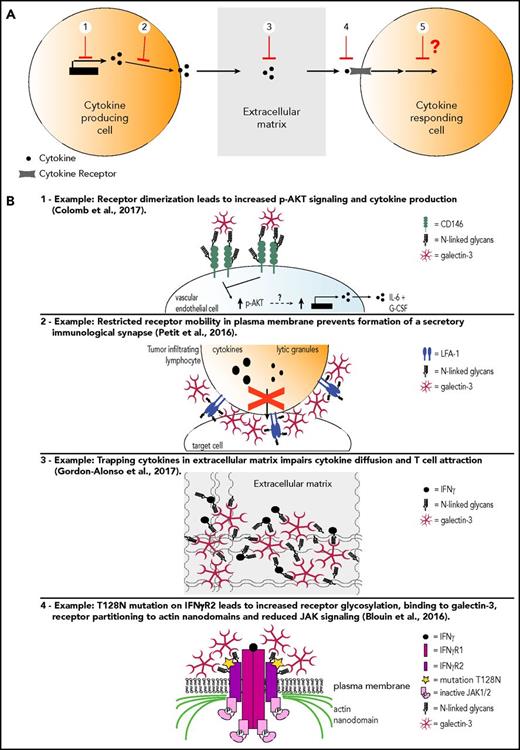
Levels of galectin regulation of cytokines. (A) 1, Cytokine transcription and production; 2, cytokine secretion; 3, cytokine diffusion in the extracellular media; 4, cytokine binding to its receptor and receptor mobility; 5, cytokine/receptor signaling. (B) Detailed examples for levels of galectin regulation of cytokines shown in panel A.
Levels of galectin regulation of cytokines. (A) 1, Cytokine transcription and production; 2, cytokine secretion; 3, cytokine diffusion in the extracellular media; 4, cytokine binding to its receptor and receptor mobility; 5, cytokine/receptor signaling. (B) Detailed examples for levels of galectin regulation of cytokines shown in panel A.
Galectins regulate the expression and secretion of cytokines
Galectin-1 induces immune tolerance through cytokine regulation, decreasing T helper 1 (Th1) and increasing Th2 cytokine levels.11,12 Galectin-1’s protective role in several autoimmune mouse models seems related to this modulation of the cytokine balance, where interleukin-5 (IL-5) and IL-10 secretion is favored and interferon-γ (IFN-γ), IL-2, IL-12, IL-17, and tumor necrosis factor-α (TNF-α) secretion is inhibited.11,13-15 This effect may be linked to galectin-1 preferentially inducing apoptosis of Th1 and Th17 lymphocyte subsets.16 In human malignancies, high galectin-1 protein expression correlates with disease progression, aggressiveness, and poor survival in chronic lymphocytic leukemia (CLL) and multiple myeloma (MM).17,18 In both cases, galectin-1 is secreted by the tumor stroma and favors tumor growth and survival. Specifically, in CLL, the galectin-1–secreting stroma is represented by nurse-like cells (NCL), a monocytic differentiated cell type.19 NCLs are known to protect the leukemic clones from spontaneous and drug-induced apoptosis.19 Knocking down galectin-1 in NCL reduces their expression of B-activating factor and the secretion of IL-10 and CCL3 by cocultured CLL B cells.17 Importantly, high serum levels of IL-10 and CCL3 correlate with shorter time to first treatment and survival of CLL patients.20 Although not evaluated by these authors, B-activating factor interactions with CD86 are known to induce IL-10 secretion by B cells.21 These NCL-CLL contacts might represent the mechanism underlying IL-10 upregulation. Furthermore, NCL-secreted galectin-1 activates survival signaling through the B-cell receptor in CLL cells.17 Galectin-1 is also a pre–B-cell receptor ligand that induces receptor clustering, which represents the first checkpoint of B-cell differentiation and may further support CLL survival.22 In MM, the expression of galectin-1 is directly induced by the bone marrow hypoxic microenvironment.18 Galectin-1, by an unexplored mechanism, increases the expression of proangiogenic CCL2 and MMP9 and reduces the expression of angiostatic SEMA3A and CXCL10.18 Consequently, injecting mice with myeloma cell lines in which galectin-1 was knocked down reduces tumor vascular density, and most likely because of this anti-angiogenic effect, also tumor growth and tumor burden.18 Interestingly, galectin-1 and -3 have important proangiogenic effects independently of cytokine regulation, mainly by binding directly to vascular endothelial growth factor receptors.23 In summary, galectin-1 and other galectins released by the tumor stroma aids the tumor by increasing the expression of protumoral cytokines, chemokines, and angiogenic factors. However, the precise mechanisms are still unknown.24
Galectin-3 is considered proinflammatory and prometastatic.3,4 The proinflammatory effect of galectin-3 has only been reported in nonhematological tumors and consists mostly of galectin-cytokine correlations. In pancreatic ductal adenocarcinoma, galectin-3 expression is higher than in normal pancreatic tissue and correlates with higher IL-8, CCL2, and CXCL1 expression.25 Increased galectin-3 is also reported in the serum of colon cancer patients where it correlates with higher granulocyte colony-stimulating factor (G-CSF), IL-6, and sICAM-1 levels.26,27 Galectin-3–related cytokines are considered prometastatic because they increase drug resistance and the adhesion and migration of cancer cell lines to and through the endothelium.26,28,29 Galectin-3 upregulates cytokine expression through the promotion of Ras/MEK pathways, leading to increased AKT survival signals and NF-κB–driven cytokine production.27,30,31 Although most studies show that extracellular galectin-3 promotes these signaling pathways, the precise mechanism of action remains unknown. Galectin-3 has been proposed to crosslink glycoprotein receptors on the cell surface, but both agonistic and antagonistic effects on downstream signaling from the receptors have been reported (eg, Markowska et al32 vs Petit et al 33 ). Galectin-3 is an important prognosis factor for poor disease progression in several leukemias (see Table 2) and has been correlated with bone marrow–induced drug resistance in CLL and AML in vitro.34,35 In summary, although galectin-3 is clearly involved in proinflammatory and prometastatic progression of cancer, its precise roles and mechanisms of action are still elusive.
Galectin-4 is secreted by healthy intestinal epithelium and human colorectal cancer cell lines and binds to the surface of mucosal lamina propria T cells and colorectal cancer cells, respectively.36,37 Colorectal cancer cell lines treated in vitro with galectin-4 neutralizing antibodies proliferate more and secrete CCL2, CCL5, CSF-2, and soluble ICAM-1 by an unknown mechanism.37 Other authors confirmed the antitumoral effect of galectin-4 by knocking down galectin-4 in preclinical tumor models.38 Interestingly, these authors observed that silencing galectin-4 was associated with higher expression of IL-6. Furthermore, galectin-4 associates with CD3, inhibits T-cell activation and proliferation upon CD3/CD28 stimulation and reduces downstream secretion of IL-6, IL-8, IL-10, and TNF-α.36 However, it is unclear if galectin-4 binding to CD3 is the root cause of the downstream T-cell dysfunction.
Galectin-9 studies yielded contradicting results. Galectin-9 protein levels are increased in AML cells and in the blood plasma of AML patients.39,40 AML progression is associated with T-cell exhaustion and dysfunction, including reduced cytokine secretion.40,41 T-cell exhaustion in AML has been linked to galectin-9 interactions with Tim-3, an inhibitory receptor upregulated at the surface of activated and exhausted T cells.40 Treating a mouse AML model with a Tim-3 human-IgG fusion protein and an anti–PD-L1 antibody reduced tumor burden and increased mouse survival rates. In addition, galectin-9 knockout mice are more resistant to AML.40 However, other authors recently described another partner of Tim-3, called CEACAM-1, which regulates Tim-3 expression and its T-cell exhaustion-inducing function.42 Hence, the therapeutic effect of blocking Tim-3 might be unrelated to galectin-9. It is noteworthy that CEACAM-1 and Tim-3 are both N-glycosylated, and thus, galectin-9 might bind both proteins, and stabilize or block their association, which has not been studied yet. A complicating factor is that the Tim-3 exists in cell surface–bound and soluble forms, following proteolytic shedding.39 Only few authors consider this.39
The effects of Tim-3 on the tumoricidal functions of NK cells are similarly inconsistent. The addition of galectin-9 to Tim-3–positive NK cells attenuates NK killing of human AML cell lines in vitro.39 In contrast, galectin-9 was shown to reduce the killing activity of human NK cells by a Tim-3–independent mechanism.43 Reports on the influence of galectin-9 on IFN-γ production are likewise contradictory.42,43
Galectins modulate cytokine signaling
Binding of galectins to glycosylated receptors modulates how cytokines engage with their receptors and the subsequent signaling pathways. For instance, the galectin-3 antagonist polysaccharide GCS-100 has been shown to inhibit responses to the cytokines TNF-α, IGF-1, and IL-6 by myeloma cell lines by a mechanism not yet identified.44 GCS-100 also reduced myeloma proliferation by promoting tumor cell apoptosis.44
Blouin and colleagues elegantly demonstrated that a mutation on IFNγR2, causing the addition of a new N-glycosylation site, leads to the complete abrogation of IFN-γ signaling.45 This gain in glycosylation reinforces the association of galectin-3 with the IFNγR2, which in turn confines IFNγR2 into membrane actin nanodomains from where it cannot signal.45 Treatment with N-glycosidase, galectin antagonists, or knocking down galectin-3 expression rescued IFNγR2 distribution into lipid nanodomains on the plasma membrane and consequently restored signaling transduction.45
Extracellular galectins restrain the diffusion of cytokines
We recently reported that galectins modulate cytokine function through a new mechanism: galectins bind glycosylated cytokines directly and consequentially retain them within the extracellular matrix. In particular, galectin-3 binds glycans on IFN-γ and IL-12, reducing their diffusion through a collagen matrix.46 Ex vivo treatment of human tumor biopsies with galectin antagonists and IFN-γ increased CXCL9 production in most samples compared with treatment with IFN-γ alone. Moreover, only in highly infiltrated samples (hot tumors), galectin inhibitors were able to induce CXCL9 production, even when IFN-γ was not exogenously added. Because we have previously shown that galectin antagonists do not induce CXCL9 per se, the inhibition of galectin-3 in hot tumors must be releasing pretrapped endogenous IFN-γ.46 In addition, galectin-3 antagonists enhanced IFN-γ–induced CXCL9/10/11 production by the tumor in a humanized melanoma mouse model. The generated chemokine gradient increased the infiltration by specific antitumor CD8 T cells and delayed tumor growth.46
Cytokines regulate galectin expression and function
Little is known about how cytokines regulate galectin expression. TNF-α and IFN-γ induce the secretion of galectin-9 by mesenchymal stem cells (MSC) in vitro.47 In fact, the authors hypothesized that the secreted galectin-9 is responsible for the MSC suppression of lymphocyte activation.47 Other authors have confirmed that galectin-9 expression is upregulated by IFN-γ and IL-10 and downregulated by IL-1.48,49
Smith and colleagues recently reported an interesting example of cytokines influencing galectin function in a nontumoral mouse model. The authors showed that during viral chronic infection, where a sustained induction of IL-10 occurs, IL-10 upregulates the expression of mgat-5, a Golgi-resident glycosyltransferase.50 Mgat-5 catalyzes the production of highly branched N-glycoproteins that are high-affinity binding sites for galectins. Consequently, the sustained presence of IL-10 during viral chronic infections increases galectin-3 binding to CD8 T-cell surface and decreases T-cell function.50 Galectin-3 binding to glycosylated receptors in human T cells has already been linked to an increased T-cell receptor activation threshold and CD8 dysfunction.33,50-52 This dysfunction is directly linked to an inability by CD8 to secrete IFN-γ, TNF-α, and IL-2.33 Thus, an interesting cytokine-galectin cross talk seems at work, where sustained presence of IL-10 increases the expression of galectin-3 ligands, and therefore, galectin-3 binding. This in turn blocks the secretion of Th1 cytokines.
Moreover, IL-4 and IL-6 have been described to regulate the expression of several glycosyltransferases and consequently alter protein glycosylation.53 However, the authors did not analyze the particular consequences of these glycosylation changes on galectin function.
Conclusion and future directions
Galectins and cytokines seem reciprocally regulated during homeostasis and disease, but most available evidence is indirect and consists of correlating protein expression levels. Further studies are thus needed to unveil the direct links between these 2 protein families. The scientists are challenged in their search for specific and direct roles, because promiscuous binding of galectins makes them pleiotropic and able to modulate the function of cytokines at multiple levels (Figure 1). How functionally redundant are galectins for cytokine regulation?
The main mechanism of action for extracellular galectins is binding and crosslinking glycosylated proteins, such as receptors, matrix proteins, and cytokines themselves.54 Therefore, the glycosylation status of the cell affects how galectin binds to its surface.16 This status is related to the metabolism and genetic program of the cell and can be altered by cytokines, which may thus directly modulate galectin expression and indirectly alter galectin binding and function by controlling the glycosylation status of the cell.50,53 Would glycosylation changes be a new way to evaluate the effects of therapeutic cytokines? Most cancer cells are known to have an aberrant glycosylation status. Is this aberrant glycosylation regulated by the cytokines present in the tumor microenvironment?
Because galectins seem also involved in tumor drug resistance (see Table 2), combinations of galectin inhibitors with current cytotoxic drugs might increase the efficiency of present treatments. We believe that galectins could be interesting therapeutic targets for 3 main reasons: they are overproduced along cancer progression, their presence correlates with poor prognosis, and they seem to regulate cytokine production and function.
Acknowledgments
The authors thank Thibault Hirsch for manuscript revision.
This review follows work supported by grant 2010-175 from the Fondation Contre le Cancer (Belgium) and grant 3.4514.12 from the Fonds de la Recherche Scientifique Médicale-FRSM (Belgium).
Authorship
Contribution: M.G.-A. and P.v.d.B. designed the manuscript; M.G.-A. wrote the manuscript; A.M.B. edited the manuscript, created the figures, and strongly contributed to writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Monica Gordon-Alonso, de Duve Institute, Université Catholique de Louvain, Avenue Hippocrate 74, Bte B1.74.03, B-1200 Brussels, Belgium; e-mail: monica.gordon-alonso@f-star.com; and Pierre van der Bruggen, de Duve Institute, Université Catholique de Louvain, Avenue Hippocrate 74, Bte B1.74.03, B-1200 Brussels, Belgium; e-mail: pierre.vanderbruggen@uclouvain.be.