Abstract
Recent studies have revealed that the intestinal bacterial microbiome plays an important role in the regulation of hematopoiesis. A correlation between adverse hematologic effects and imbalance of the intestinal microbiome, or dysbiosis, is evident in several human conditions, such as inflammatory bowel disease, obesity, and, critically, in the setting of antibiotic exposure. Here we review the effects of gut dysbiosis on the hematological compartment and our current understanding of the mechanisms through which changes in the bacterial microbiome affect hematopoiesis.
Introduction
The microbiome influences many biological processes, from early brain development to aging of innate immune cells.1-5 Several recent studies have demonstrated that the bacterial microbiome also plays an important role in normal hematopoiesis.6-9 Human conditions associated with altered intestinal bacterial populations, such as inflammatory bowel syndrome or prolonged antibiotic use, are associated with adverse hematologic effects, including anemia and neutropenia. Understanding the mechanisms by which the microbiome influences normal blood production may help with development of novel treatment modalities to prevent these complications. Here, we discuss the interactions between the microbiome and hematopoiesis, review what is currently known about the mechanisms underlying these connections, and propose a model of signaling between the gut microbiome and bone marrow (BM).
The impact of microbiota depletion on hematopoiesis in mice
The term microbiome describes the diverse array of microorganisms, including bacteria, viruses, fungi, and archaea, that colonize the human body, forming an ecological system critical to human health.5 Much of what is currently known about the connection between microbiota and hematopoiesis is derived from murine studies. For example, microbiota are entirely lacking in germ-free (GF) mice, and these mice have well-known abnormalities in BM cell populations.6,7,9-11 GF mice have smaller hematopoietic stem and progenitor cell (HSPC) populations, abnormal splenic myeloid counts, and impaired T-cell function compared with their specific-pathogen-free (SPF) counterparts.7,9,10 Similarly, oral antibiotics deplete intestinal bacteria and have suppressive effects on hematopoiesis. Adult SPF mice treated with antibiotics for 1 week or more develop BM suppression, and these effects are largely independent of treatment duration, absorption, and type of antibiotic used.2,7,8,11 Antibiotics have also been shown to disrupt engraftment of HSPCs after transplantation in mice, indicating that the microbiome plays an important role in the posttransplant setting.12 Importantly, biologically relevant concentrations of antibiotics are not toxic to HSPCs under in vitro culture conditions, arguing against a direct antibiotic effect on hematopoiesis.8 Instead, several recent studies, summarized in detail in Table 1, indicate that antibiotics impair normal hematopoiesis by depleting intestinal bacteria. Many of the features of hematopoietic suppression related to microbiota depletion in mice, including timing, are similar to those seen in humans, as we describe below.
Mechanisms of microbiota and host communication to regulate hematopoiesis
| Reference . | Mouse model . | Antibiotics . | Treatment duration, wk . | Microbiota mechanism . | Host mechanism . |
|---|---|---|---|---|---|
| 7 | GF/ABX | Vancomycin | 4-5 | MAMPs | Not discussed |
| Ampicillin | |||||
| Neomycin | |||||
| Streptomycin | |||||
| 6 | GF/ABX | Vancomycin | 4 | Heat-killed microbial product | Signaling through MyD88/TICAM1 |
| Ampicillin | |||||
| Neomycin | |||||
| Metronidazole | |||||
| 8 | ABX | Vancomycin | 2 | Not discussed | Signaling through STAT1 |
| Ampicillin | |||||
| Neomycin | |||||
| Metronidazole | |||||
| 9 | GF | N/A | NOD1 ligand | Production of HSPC proliferation-stimulating cytokines by MSCs | |
| N/A | |||||
| Reference . | Mouse model . | Antibiotics . | Treatment duration, wk . | Microbiota mechanism . | Host mechanism . |
|---|---|---|---|---|---|
| 7 | GF/ABX | Vancomycin | 4-5 | MAMPs | Not discussed |
| Ampicillin | |||||
| Neomycin | |||||
| Streptomycin | |||||
| 6 | GF/ABX | Vancomycin | 4 | Heat-killed microbial product | Signaling through MyD88/TICAM1 |
| Ampicillin | |||||
| Neomycin | |||||
| Metronidazole | |||||
| 8 | ABX | Vancomycin | 2 | Not discussed | Signaling through STAT1 |
| Ampicillin | |||||
| Neomycin | |||||
| Metronidazole | |||||
| 9 | GF | N/A | NOD1 ligand | Production of HSPC proliferation-stimulating cytokines by MSCs | |
| N/A | |||||
ABX, antibiotics; MAMPs, microbe-associated molecular patterns; MSCs, mesenchymal stromal cells; N/A, not applicable.
Suppression of hematopoiesis associated with dysbiosis in humans
Dysbiosis, or imbalance, of the gut microbiome has been linked to suppression of hematopoiesis in humans. Note that the term dysbiosis does not refer to the presence of specific microbial pathogens, which can certainly exert hematologic effects as recently reviewed.13 An imbalance in commensal intestinal bacteria characterizes inflammatory bowel diseases (IBDs), including decreased bacterial diversity, enhanced bacteriophage populations, and outgrowth of pathobionts.14,15 Interestingly, IBD has been independently linked to non-drug-induced aplastic anemia.16,17 A murine model of IBD showed that intestinal inflammation exerts significant stress on HSPCs.18 Nutritional disorders also share a link between dysregulated microbial communities and altered hematological outcomes. For instance, obesity has been associated with both dysbiosis19-21 and hematopoietic abnormalities in humans22,23 and mice.24,25 Malnutrition also is associated with altered intestinal microbial communities,26,27 as well as frequently severe hematological disturbances.28,29 Although these complex conditions in humans have many potential confounding factors, including an altered inflammatory milieu, cellular access to nutrients, and genetic factors, they highlight the potential for microbial dysbiosis and hematological abnormalities to be linked by association or perhaps causally.
A more direct link between microbial dysbiosis and hematopoietic alterations can be observed in patients receiving antibiotics. Antibiotic treatment, which causes gut dysbiosis by eliminating certain classes of bacteria, is widely associated with hematological abnormalities. Cytopenias, including neutropenia, anemia, thrombocytopenia, and pancytopenia, have been reported for a wide range of antibiotics.30-35 For example, a retrospective analysis found that 5% to 15% of patients developed neutropenia (defined as <1000 neutrophils per cubic millimeter) after 10 or more days of β-lactam antibiotic treatment.36 Of the patients that developed neutropenia, 94% recovered neutrophil counts after stopping antibiotic treatment. This finding was corroborated in a review of published clinical case reports that showed patients developed neutropenia when treated with penicillin G.30 These studies suggest that disrupting the gut microbiome through antibiotic use may have a significant impact on hematopoiesis.
Importantly, hematologic abnormalities due to antibiotics are not limited to a single class of antibiotics.30,32-40 Indeed, neutropenia was one of the most common adverse drug-related effects of outpatient parenteral antimicrobial therapy (OPAT) in pediatric patients regardless of the antibiotic agent used.41-44 Confirming this finding, Fernandes et al45 found that leukopenia developed in 6% of pediatric patients on OPAT for a median of 30 days. Of note, the antibiotic trimethoprim-sulfamethoxazole (TMP-SMZ) is widely known to cause neutropenia, but the rate of TMP-SMZ–mediated neutropenia is far lower than that reported for other antibiotics, such as β-lactams. Indeed, the incidence of blood disorders associated with TMP-SMZ (5.6 cases per 100 000 patients) is ∼1000 times lower than that of prolonged antibiotics as described in the OPAT studies mentioned above.46-48 Trimethoprim has been shown to exert an antifolate activity on granulocyte progenitors, an effect that can be reversed by folinic acid administration.49 However, cytopenias related to other antibiotics have not been related to folate deficiency, and their vastly different incidence suggests that the mechanisms of action differ as well.
Similar to the results of recent murine studies, antibiotic treatment can affect the outcome of allogenic hematopoietic stem cell transplant in humans.50-54 Despite the common use of antibiotics following BM transplant, these studies indicate that the reduction in diversity and abundance of intestinal microbiota resulting from antibiotic administration impairs engraftment and increases the risk of leukemic relapse, graft versus host disease, and death following transplant.12,53,55
In summary, syndromes of dysbiosis are linked to hematologic defects, and 5% to 15% of patients on long-term antibiotic treatment are vulnerable to adverse hematologic complications. Antibiotic courses lasting >2 weeks, when hematologic effects become more common, can be appropriate for many conditions, including osteomyelitis, endocarditis, septic arthritis, and meningitis.43 However, antibiotic-associated neutropenia leaves patients vulnerable to opportunistic and potentially fatal infections, and weekly monitoring for evidence of antibiotic-associated cytopenias is both costly and painful. Stopping or changing antibiotics due to adverse effects adds additional costs and hinders effective treatment, especially for HSCT patients. Thus, understanding the mechanistic basis linking antibiotic use, gut dysbiosis, and hematologic abnormalities is an important clinical priority.
Mechanisms of long-term antibiotic-induced suppression of hematopoiesis
Although long-term antibiotic treatment can clearly affect hematopoiesis, the mechanisms of suppression remain contentious. Several early studies suggested that β-lactam antibiotics directly suppress the differentiation of progenitor cells36,56 ; however, those findings were based on in vitro studies that only showed inhibition at half maximal inhibitory concentration of 100 to 600 μg/mL. These concentrations are much higher than those typically achieved in humans (<50 μg/mL),57 thus calling into question the clinical and biological relevance of these findings. To assess mechanisms of suppression at antibiotic concentrations in the range of those expected in vivo, we cocultured murine BM with the β-lactam antibiotic ceftriaxone (25 μg/mL) or with vancomycin, neomycin, ampicillin, and metronidazole (12.5 μg/mL for vancomycin; 25 μg/mL for the rest) in methylcellulose and showed there was no effect on colony formation.8 These studies indicate that antibiotics at concentrations in the range of those expected in vivo do not antagonize progenitor activity directly.
Conversely, some studies suggested that hematopoietic suppression in patients on long-term antibiotic treatment is caused by indirect, immune-mediated mechanisms.58-60 An antibody-mediated mechanism for vancomycin-associated thrombocytopenia has been established.61 Similarly, detection of antineutrophil and antipenicillin immunoglobulin G antibodies in the sera of neutropenic patients led to the speculation that neutropenia could be a result of antipenicillin antibodies coating penicilloylated neutrophils or opsonization of immune complex coated neutrophils.59,60 Despite these reports, subsequent studies have shown that such effects do not worsen with repeated courses of penicillin, arguing against an antibody-mediated phenomenon.62
More recently, several groups independently identified changes in the microbiome as a key regulator of normal hematopoiesis (see Table 1).6-9 Suppression of granulocyte and monocyte numbers in GF mice or in antibiotic-treated mice was shown to be rescued by oral provision of MAMPs from the gut microbiota or by recolonization with normal microbiota.6,7 Consistent with these findings, a heat-resistant component of Escherichia coli in serum was found to restore BM myeloid cell populations in GF mice. Furthermore, Iwamura et al9 reported that nucleotide-binding oligomerization domain-containing protein 1 ligand (NOD1L), which is a heat-stable component of the peptidoglycan structure of E coli,63,64 increased systemic levels of HSPC proliferation-stimulating cytokines such as stem cell factor and thrombopoietin to levels found in SPF mice. These growth cytokines are produced in large part by MSCs in the BM niche. In summary, the field is converging on a paradigm in which products of the intestinal microbiota such as NOD1L can enter the bloodstream and travel to the BM, where they promote the production of growth cytokines that support normal hematopoiesis.
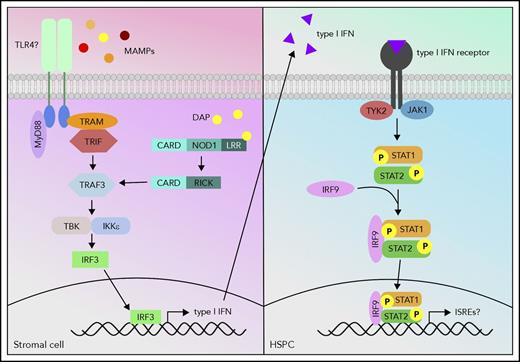
Although progress has been made in determining the signals produced by intestinal microbes to trigger normal hematopoiesis, the host cells and receptors by which those signals are detected have not yet been identified. Treatment of MyD88−/−TICAM1−/− GF mice with heat-inactivated serum from SPF mice did not expand the BM myeloid compartment,6 suggesting that MyD88 and TICAM1 are required for appropriate gut-marrow signaling. Our recent study showed that Myd88 single knockout mice had no apparent defect in antibiotic-mediated BM suppression, indicating that MyD88 and TICAM1 may have redundant roles.8 Meanwhile, Stat1−/− mice had BM HSPC and granulocyte counts as low as antibiotic-treated wild-type mice, and treating Stat1−/− mice with antibiotics did not further suppress cell counts, suggesting that STAT1 signaling stimulated by the microbiota is required for normal hematopoiesis.8 Indeed, these data fit into a growing narrative that basal inflammatory signaling is required to maintain normal hematopoiesis.8,65 Recently, Iwamura et al9 determined that NOD1−/− MSCs, in contrast to wild type, are unable to produce HSPC proliferation-stimulating cytokines, indicating that NOD1 signaling through MSCs is an important regulator of hematopoiesis.9 Therefore, it is likely that MyD88/TICAM1, NOD1, and STAT1 are all involved in regulating steady-state hematopoiesis; in fact, it is possible that they all feed into the same pathway. The MyD88-dependent Toll-like receptor (TLR) pathway and NOD1 pathway share similar downstream signaling molecules such as TRAF3, which signals to IRF3 to induce interferon production, and interferons signal via STAT1. Further studies are necessary to confirm the interplay of these proposed signaling pathways (Figure 1) in microbiota-mediated hematopoiesis.
Proposed model of host signaling cascade in response to microbial signals to promote hematopoiesis. MAMPs activate the TLR pathway, and meso-diaminopimelic acid (DAP) activates the NOD1 pathway in stromal cells. The TLR and NOD1 pathways can cross talk at tumor necrosis factor receptor-associated factor 3 (TRAF3) and induce type I interferon (IFN) production. These type I IFNs can then activate the type I IFN pathway via signal transducer and activator of transcription 1 (STAT1) in HSPCs and activate a gene profile that is necessary to promote hematopoiesis.
Proposed model of host signaling cascade in response to microbial signals to promote hematopoiesis. MAMPs activate the TLR pathway, and meso-diaminopimelic acid (DAP) activates the NOD1 pathway in stromal cells. The TLR and NOD1 pathways can cross talk at tumor necrosis factor receptor-associated factor 3 (TRAF3) and induce type I interferon (IFN) production. These type I IFNs can then activate the type I IFN pathway via signal transducer and activator of transcription 1 (STAT1) in HSPCs and activate a gene profile that is necessary to promote hematopoiesis.
Conclusions
In summary, gut dysbiosis is associated with hematological abnormalities in both humans and mice. Murine studies now show that antibiotic-induced microbiota depletion and BM suppression are due to the absence of heat-stable microbial products that can circulate in the bloodstream and promote hematopoiesis through basal inflammatory signaling. These mechanisms are potentially significant in many patients, including those requiring long-term antibiotic therapy and those recovering from HSCT.
Although studies have begun to uncover the mechanisms of antibiotic-induced hematological adverse effects, many details remain unknown and new questions arise. The range of microbial products that signal to the host to promote normal hematopoiesis and the microbial species from which they derive still need to be elucidated.66-68 Balmer et al6 found that the TLR pathway components MyD88 and TICAM1 are required for steady-state granulopoiesis, but stopped short of determining which microbial product was responsible for activating the TLR signaling. Some microbial metabolites such as short-chain fatty acids have been shown to contribute to the production of hematopoietic precursors in SPF mice,69 but other microbial metabolites, such as from indole or flavonoid metabolism,70,71 have not been studied. In addition, the tissues necessary to transmit signals from gut microbes to hematopoietic progenitors remain unknown. Future research will broaden our understanding of the role microbiota play in regulating hematopoiesis and may identify therapeutic interventions to support healthy hematopoiesis in patients with gut dysbiosis.
Acknowledgments
The authors would like to thank C. Gillespie for critical reading of the manuscript.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL136333 and R01HL134880 (K.Y.K. and H.Y.), the Aplastic Anemia and MDS International Foundation Liviya Anderson Award (K.Y.K.), and a March of Dimes Basil O’Connor Starter Scholar Award (K.Y.K.). M.T.B. was supported by the Global Probiotics Council’s Young Investigator Grant for Probiotics Research. H.Y. is supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant T32 DK060445.
Authorship
Contribution: H.Y. wrote the review and made the figures; and M.T.B. and K.Y.K. revised and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katherine Y. King, Pediatric Infectious Diseases, Baylor College of Medicine, Feigin Center for Pediatric Research, 1102 Bates Ave, Suite 1150, Houston, TX 77030; e-mail: kyk@bcm.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal