Abstract
Chimeric antigen receptor T cells demonstrate efficacy in B-cell malignancies, leading to US Food and Drug Administration approval of axicabtagene ciloleucel (October 2017) and tisagenlecleucel (May 2018) for large B-cell lymphomas after 2 prior lines of therapy. Durable remissions are seen in 30% to 40% of study-treated patients, but toxicities of cytokine release syndrome and neurotoxicity require administration in specialized centers. This article reviews data of current diffuse large B-cell lymphoma management, focusing on axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel.
Introduction
Diffuse large B-cell lymphoma (DLBCL) and other aggressive B-cell malignancies are typically fatal in patients not cured with initial therapy.1-4 For medically fit patients with chemosensitive disease, high-dose therapy followed by autologous stem cell transplant (ASCT) can offer long-term remissions for a minority; however, most patients with relapsed or refractory (R/R) DLBCL will die of lymphoma.3 Cellular immunotherapy may eradicate B-cell malignancies, with early evidence from allogeneic hematopoietic stem cell transplantation.5 More recently, the ability to transduce and expand CD3+ chimeric antigen receptor (CAR) T cells ex vivo have allowed for specific tumor antigen targeting and adequate signaling for in vivo proliferation and resultant antitumor responses. Recent results in this field have led to 3 US Food and Drug Administration (FDA) approvals in B-cell malignancies, with more approvals anticipated.6-8 Herein we provide an overview of the key data and practical information to date for the use of anti-CD19 CAR T cells in treating R/R DLBCL and other aggressive B-cell lymphomas.
Anti-CD19 CAR T-cell construct and production
Common features of CAR T-cell therapy include collection of a patient’s T cells via leukapheresis, variable selection and activation of the T cells, transduction of the anti-CD19 CAR gene using a retroviral or lentiviral vector, expansion of the final product in vitro, and infusion of the product back to the patient.9,10 Lymphodepleting chemotherapy prior to cellular infusion leads to endogenous cytokine stimulation promoting CAR T-cell expansion and proliferation.11-13 Infusion is typically performed in centers experienced in hematopoietic stem cell transplantation because they are most equipped to manage unique toxicities including cytokine release syndrome (CRS) and neurotoxicity (NT). Successful outpatient administration of CAR T cells can be accomplished and is reliant on the product, institutional infrastructure, and may be trial dependent.14 “Second-generation” CAR T cells fuse together an extracellular antigen-recognizing domain consisting of a single-chain variable fragment derived from a monoclonal antibody recognizing a specific tumor antigen (eg, CD19), a hinge/transmembrane region, and an intracellular T-cell signaling component consisting of a CD3ζ subunit T-cell activating domain with a costimulatory domain (CD28 or 4-1BB).9,10 Table 1 lists key features of the 3 second-generation anti-CD19 CAR T-cell products available commercially or in late clinical trials. Manipulating these key elements may facilitate expansion in vivo, alter toxicity profiles, and possibly overcome disease resistance and persistence.15
Composition, efficacy, and safety comparisons
| . | Axicabtagene ciloleucel . | Tisagenlecleucel . | Lisocabtagene maraleucel . |
|---|---|---|---|
| Study populations | DLBCL, TFL, PMBCL | R/R DLBCL | CORE DL2* |
| Target antigen | CD19 | CD19 | CD19 |
| Lymphodepletion | Flu/Cy | Flu/Cy | Flu/Cy |
| Costimulatory domain | CD28 | 4-1BB | 4-1BB |
| T-cell composition | Unspecified | Unspecified | 1:1 CD4:CD8 |
| Cell dose | 2 × 106 cells/kg | 5 × 108 | 1 × 108 |
| OR (best) | 82% (N = 108) | 53% (N = 81) | 81% (N = 27) |
| OR (6 mo) | 41% (N = 101) | 37% (N = 46) | 50% (N = 14) |
| CR (best) | 58% (N = 108) | 40% (N = 81) | 63% (N = 27) |
| CR (6 mo) | 36% (N = 101) | 30% (N = 46) | 50% (N = 14) |
| Any grade CRS/NT† | 94%/87% (N = 108) | 58%/21% (N = 99) | 24%/17% (N = 29) |
| ≥Grade 3 CRS† | 12% (N = 108) | 23% (N = 99) | 0% (N = 29) |
| ≥Grade 3 NT† | 31% (N = 108) | 12% (N = 99) | 7% (N = 29) |
| Grade 5 AEs | 4% (N = 108)‡ | None | — |
| . | Axicabtagene ciloleucel . | Tisagenlecleucel . | Lisocabtagene maraleucel . |
|---|---|---|---|
| Study populations | DLBCL, TFL, PMBCL | R/R DLBCL | CORE DL2* |
| Target antigen | CD19 | CD19 | CD19 |
| Lymphodepletion | Flu/Cy | Flu/Cy | Flu/Cy |
| Costimulatory domain | CD28 | 4-1BB | 4-1BB |
| T-cell composition | Unspecified | Unspecified | 1:1 CD4:CD8 |
| Cell dose | 2 × 106 cells/kg | 5 × 108 | 1 × 108 |
| OR (best) | 82% (N = 108) | 53% (N = 81) | 81% (N = 27) |
| OR (6 mo) | 41% (N = 101) | 37% (N = 46) | 50% (N = 14) |
| CR (best) | 58% (N = 108) | 40% (N = 81) | 63% (N = 27) |
| CR (6 mo) | 36% (N = 101) | 30% (N = 46) | 50% (N = 14) |
| Any grade CRS/NT† | 94%/87% (N = 108) | 58%/21% (N = 99) | 24%/17% (N = 29) |
| ≥Grade 3 CRS† | 12% (N = 108) | 23% (N = 99) | 0% (N = 29) |
| ≥Grade 3 NT† | 31% (N = 108) | 12% (N = 99) | 7% (N = 29) |
| Grade 5 AEs | 4% (N = 108)‡ | None | — |
Courtesy of and adapted from C. Turtle.
AE, adverse event; Cy, cyclophosphamide; DL2, dose level 2; Flu, fludarabine; OR, overall response.
CORE group (proposed pivotal population) including DLBCL, not otherwise specified TFL, FL3B, ECOG 0-1, and R/R patients.
CAR T toxicity grading scales differ across studies.
2 patients grade 5 CRS.
Axicabtagene ciloleucel (axi-cel, Yescarta) received FDA approval in October 2017 for R/R lymphoma based on results from the Phase 1-2 Multi-Center Study Evaluating Axicabtagene Ciloleucel in Subjects With Refractory Aggressive Non-Hodgkin Lymphoma (ZUMA-1) trial. It was the first anti-CD19 CAR T-cell product indicated for DLBCL, transformed follicular lymphoma (TFL), primary mediastinal large B-cell lymphoma (PMBCL), and high-grade B-cell lymphoma after at least 2 prior lines of therapy. A total of 101 patients with refractory DLBCL, TFL, or PMBCL, defined as failure to last chemotherapy or relapse ≤12 months following ASCT, received 2 × 106 CAR+ cells/kg. Lymphodepletion consisted of cyclophosphamide 500 mg/m2 and fludarabine 30 mg/m2 for 3 days. No bridging chemotherapy was allowed during the median 17 days between collection and infusion. At a median follow-up of 15.4 months, the best overall response rate (ORR) was 82%, with 58% meeting criteria for complete response (CR). Ongoing responses were observed in 42%, with durable remissions seen for those achieving an early CR. Estimated progression free survival was 41% at a median follow-up of 15 months. Median overall survival (OS) had not been reached at time of data cutoff.6
Tisagenlecleucel (Kymriah) received FDA approval for DLBCL, TFL, and high-grade B-cell lymphoma after at least 2 lines of therapy on May 1, 2018. Previously, it was only approved for R/R precursor B-cell acute lymphoblastic leukemia in patients ≤25 years of age. Schuster et al first published their results on 38 patients with R/R CD19+ non-Hodgkin lymphoma (NHL) from the University of Pennsylvania, 28 of whom received a median of 5.79 × 106 CAR+ cells/kg.16 Lymphodepletion was at the discretion of the treating physician. A median 39 days elapsed between collection and infusion. Bridging chemotherapy was allowed. At a median follow-up of 28.6 months, the DLBCL cohort achieved ORR 50% and CR 43%.16 The Study of Efficacy and Safety of CTL019 in Adult DLBCL Patients (JULIET) trial then brought tisagenlecleucel globally, replicating the data that served as the basis for FDA approval.8 A total of 147 patients from 27 countries with refractory DLBCL or TFL were enrolled, 99 infused, and 81 evaluated. The best ORR and CR were 53% and 40%, respectively, with durable responses noted between 3 and 6 months. Median duration of response and OS had not been reached, but the probability of being relapse free at 6 months was 74%, translating into ∼40% of patients being without disease progression.8
Lisocabtagene maraleucel (JCAR017) is formulated at a specified CD4+:CD8+ composition and administered in a flat dose. The ongoing phase 1 Study Evaluating the Safety and Pharmacokinetics of JCAR017 in B-cell Non-Hodgkin Lymphoma (TRANSCEND-NHL-001) enrolled patients with R/R DLBCL, PMBCL, FL3B, or mantle cell lymphoma.14 After administering JCAR017 in 3 doses for the initial dose-finding cohorts, dose level 1 5 × 107 and dose level 2 1 × 108 CAR+ cells were selected for the expansion cohorts.14 Lymphodepletion comprised of cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 for 3 days. The DLBCL cohort was further subdivided into a CORE cohort and a FULL cohort. The CORE cohort included high-grade B lymphoma (double/triple hit) and DLBCL not otherwise specified (de novo, TFL). The FULL cohort included CORE patients in addition to DLBCL transformed from chronic lymphocytic leukemia, marginal zone lymphoma, PMBCL, and FL3B, as well as those who had received prior ASCT (N = 5) or had an Eastern Cooperative Oncology Group (ECOG) performance status of 2 (N = 10). A total of 68 patients were evaluated, with best ORR and 3- and 6-month ORR reported as 75%, 49%, and 40%. The best CR rate and CR rate at 3 and 6 months were 56%, 40%, and 37%, respectively. Enrollment for the pivotal DLBCL cohort is using dose level 2 based on an improved trend in response.
Safety
CRS
CRS is characterized by fevers, hypoxia, hypotension and, rarely, multiorgan failure or evolution of hemophagocytic lymphohistiocytosis.17 Following engagement of the CAR T cell to its target antigen, T-cell activation promotes cytokine release, mediating symptoms of CRS.18 Onset commonly occurs within the first week, peaking 1 to 2 weeks after administration, but may differ according to the product. ZUMA-1, JULIET, and TRANSCEND identified median onsets of 2, 3, and 5 days with axi-cel, tisagenlecleucel, and JCAR017, respectively.6,8,14 Severity ranged from 0% to 23% for any CRS ≥3 based on available preliminary datasets.6,8,14 CRS grading is largely based on criteria proposed by Lee et al, with severity driving treatment.17 This includes supportive, floor-level care to aggressive, intensive care unit (ICU) care with additional anti–interleukin-6 therapies (eg, tocilizumab) and/or systemic corticosteroids. Of importance, the severity of this grading scale is generally higher per grade than most adverse events on the National Cancer Institute Common Terminology Criteria for Adverse Events Scale. For example, a patient requiring high-dose or multiple pressors would be scored as grade 3 CRS. Similarly, fraction of inspired oxygen up to 40% or low-dose pressor requirement would be scored as grade 2 per the Lee criteria. Furthermore, CRS grading in JULIET used the Penn Grading System, a novel criteria per Porter et al18 ; therefore, cross-trial comparisons to identify potential clinical differences in the 3 constructs are challenging because no standardized grading scales were implemented, and timing and use of corticosteroids and anti–interleukin agents varied.
Neurotoxicity
Neurotoxicity, also referred to as CAR T cell–related encephalopathy syndrome, remains poorly understood. Hypotheses include diffusion of inflammatory cytokines through the blood–brain barrier, direct central nervous system (CNS) toxicity by the engineered T cells, and endothelial dysfunction of the blood–brain barrier.19 Symptoms may be subtle (somnolence, confusion) or striking (aphasia, encephalopathy, seizures, cerebral edema).20 Treatment depends on severity, graded per National Cancer Institute Common Terminology Criteria for Adverse Events criteria. This ranges from supportive care and early diagnostic studies to aggressive, ICU-level care with antiepileptic drugs, high-dose corticosteroids, and specific interventions for status epilepticus and cerebral edema. The severity of NT may also differ by product. ZUMA-1 reported a ≥grade 3 NT of 31%, tisagenlecleucel 12%, and JCAR017 7%.6,8,14
In total, 43% and 27% of ZUMA-1 patients, 15% and 11% of JULIET patients, and 12% and 16% of JCAR017-treated patients in the FULL cohort of TRANSCEND received tocilizumab and corticosteroids, respectively.6,8,14 These unique toxicities warrant management at specialized centers with experienced providers. Other complications include infections, cytopenias, and B-cell aplasia requiring intravenous immunoglobulin infusions.6,8,14,16,21 Future data may help elucidate predictors of severity, onset, and duration of these unique toxicities. Table 1 also compares efficacy and safety data among the 3 products reviewed.
Anti-CD19 CAR T-cell therapy in clinical practice
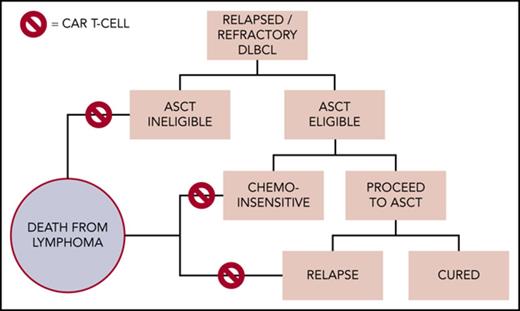
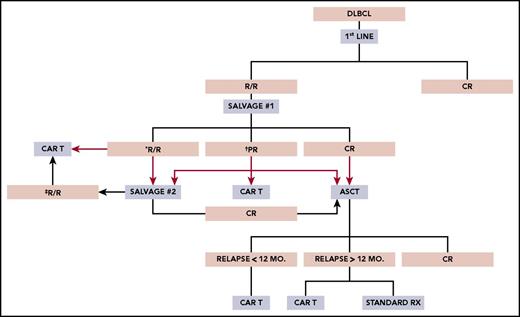
At least 3 major clinical scenarios merit consideration for CAR T-cell therapy in DLBCL, but patients must also have an ECOG grade 0-1, no major comorbidities, and no history of CNS pathology (seizures, ischemia, bleeding, dementia). Salvage platinum-based chemotherapy followed by high-dose therapy and ASCT remains the accepted treatment of transplant-eligible patients with R/R DLBCL; however, only one-half meet criteria for this approach.22 Of these patients, ∼50% will manifest chemosensitive disease and proceed with transplant; 40% who undergo transplant may be cured.2 For the remainder of patients, further curative options are limited. Figure 1 proposes a schema for CAR T-cell therapy in clinical practice, although prospective intent-to-treat data are needed to help define their use in these settings.
Proposed schema for use of anti-CD19 CAR T-cell therapy in clinical practice. PR, partial response; Rx, treatment. *Proceed to salvage 2 or CAR T-cell therapy. †Proceed to salvage 2, CAR T-cell therapy, or ASCT. ‡Proceed to CAR T-cell therapy ± salvage 3.
Proposed schema for use of anti-CD19 CAR T-cell therapy in clinical practice. PR, partial response; Rx, treatment. *Proceed to salvage 2 or CAR T-cell therapy. †Proceed to salvage 2, CAR T-cell therapy, or ASCT. ‡Proceed to CAR T-cell therapy ± salvage 3.
Post-ASCT relapse
Recurrent DLBCL within 1 year post-ASCT represents a straightforward clinical scenario for CAR T-cell therapy because outcomes for such individuals are typically dismal. Data from the pivotal trial of axi-cel indicated a 39% ORR at 1 year in patients relapsing within 1 year post-ASCT.6 However, its role in patients relapsing beyond 1 year is less clear, taking into account the somewhat favorable outcome in this group with standard therapies. Prior data have reported a median OS between 12.6 months and 5.9 years in patients relapsing beyond 1 year of ASCT, although the numbers in these series are small.3,23,24 Nevertheless, based on the data to date, one would expect improved long-term outcomes for most patients with post-ASCT relapse who are able to receive CAR T-cells.
Lack of response to first salvage
For patients not achieving CR or partial response following first salvage, high-dose therapy followed by ASCT is generally not offered immediately. Follow-up data from the Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) study indicated a 47.6% ORR and a 33.1% CR rate after second salvage in patients who failed to proceed with BEAM + ASCT.25 In addition, patients achieving a CR to second salvage experienced a 1-year OS of 70%, increasing to 88.4% after transplant.25 These data should be taken into context; however, in light of the ORCHARRD study noting lower ORR (38% to 42%) to first salvage compared to CORAL (62.8%-63.5%), likely reflecting the absence of rituximab as front-line therapy in 38% of CORAL-treated patients.3,26 Therefore, with the CAR T-cell ORR data described previously, receiving CAR T-cells on-label should certainly be considered vs second salvage, with the goal to achieve a CR followed by transplant. Practically speaking, because nonresponding patients often require urgent treatment, third-line salvage still may be needed in this setting, with the final decision to offer transplant or CAR T cells based on achievement of CR.
Partial response to first salvage
This area remains controversial for CAR T-cell use. Patients in this category represent ∼25% of those undergoing platinum-based salvage and have received 2 prior lines of therapy and still have active malignancy, justifying the use of approved CAR T cells.4 Retrospective data indicate for DLBCL patients achieving partial response based on computed tomography and a Deauville score of 4 after first salvage who undergo ASCT, the expected survival at 3 to 4 years is ∼55% (vs 75%-85% for CR).27,28 Furthermore, over one-third of these patients are expected to achieve a CR following second salvage with the previously mentioned excellent outcomes after transplant.25 Prospective randomized trials may address the utility of anti-CD19 CAR T cells in this setting.
Implementation considerations
Timing and logistics
Although targeted for DLBCL and other aggressive B-cell malignancies following 2 prior lines of therapy, CAR T-cell therapy may be viable for only a small subset of selected patients. Timely referral is paramount because patient characteristics, disease characteristics, and logistics contribute to the success or failure of receiving therapy. Patient characteristics may include CNS pathology, organ function, and declining performance status. Disease characteristics largely center on the rapidity of progression and urgency of treatment. Logistics should account for the referral process, the 2 to 3 weeks between leukapheresis to infusion, and insurance coverage. Some of these barriers are highlighted by the proportion of patients not receiving product in ZUMA-1 and JULIET even after enrollment, 9% (10/111) and 33% (48/147), respectively.6,8
Financial implications
Reimbursement for CAR T-cell therapy is complicated by its novelty and cost. Axi-cel and Kymriah cost $373 000 for a 1-time infusion, not accounting for expenses associated with hospitalizations, ICU-stays and treatment of toxicities.29 The Centers for Medicaid & Medicare Services recently settled on reimbursement rates for outpatient administration, and most private carriers are making coverage decisions on a case-by-case basis; however, standardized coding procedures and payment related to hospital admissions are critical as commercial products become more readily available.
Conclusions
Anti-CD19 CAR T-cell therapy shows promise for R/R DLBCL and other aggressive B-cell lymphomas, in which curative options are limited. Data from the ZUMA-1, JULIET, and TRANSCEND studies are reporting ORR of 50% to 80% and CR rates of 30% to 50%, leading to the FDA approval of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) within the past year. Although no head-to-head trials have compared the 3 products reviewed in this article, more individuals will inquire for and receive them at designated centers. Prospective trials are needed to help elucidate the role of anti-CD19 CAR T-cell therapy in various scenarios as described in this article, and ongoing studies are addressing its use in earlier-line settings including those with primary refractory disease or early relapse. Meanwhile, current single-arm data on long-term efficacy, safety, ease of administration, and total cost should ultimately drive the decision-making regarding use of 1 product over another, with the goal to identify which construct may be optimally suited for specific patient populations.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Cancer Institute (5K24CA184039, T32CA009515) and donations from Frank and Betty Vandermeer and Sonya and Tom Campion.
Authorship
Contribution: V.A.C., M.S., and A.K.G. wrote and edited the manuscript.
Conflict-of-interest disclosure: M.S. provides consultancy for Abbvie, Genentech, and Sound Biologics; is on the advisory board for Abbvie, Genentech, and Verastem; and receives research funding from Mustang Biopharma, Pharmacyclics, Gilead, Genentech, TG Therapeutics, Bigene, Acerta, Emergent, and Merck. A.K.G. reports grants and nonfinancial support from Teva, Bristol-Myers Squibb, Merck, Takeda, TG Therapeutics, and Effector; grants, personal fees, and nonfinancial support from Seattle Genetics, Pfizer, Janssen, Gilead, Spectrum, and Incyte; and personal fees from Aptevo, BRIM Bio, Seattle Genetics, and Sanofi. V.A.C. declares no competing financial interests.
Correspondence: Ajay K. Gopal, Seattle Cancer Care Alliance, 825 Eastlake Ave E, Mailstop G3-200, Seattle, WA 98109-1023; e-mail: agopal@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal