Abstract
Introduction: Patients with Acute Myeloid Leukaemia (AML) who harbour a FLT3-ITD mutation have a worse prognosis characterised by increased early relapse. Outcomes following relapse in such patients are typically poor, not only because of earlier relapse, but also because of worse performance post-relapse than those who have the same remission duration but are FLT3-ITD WT. In a single centre retrospective study among relapsed patients, Ravandi et al [1] found a remission rate of 24% and median survival of only 13 weeks. There are therefore twin challenges in this population: first to reduce the early relapse rate, and also to develop more effective treatments post relapse. In the recent QUANTUM-R trial [2] for patients with relapsed or refractory disease, single agent quizartinib (AC220) was found to significantly improve median survival from 20.4 weeks to 27 weeks when compared to "doctor's choice" treatment (low-dose ara-C, MEC or FLAG-Ida). There is however, no directly comparable data for the population in the QUANTUM-R trial. To contextualise these results, especially given the potentially different outcomes by control treatment, we looked at outcomes in the UK NCRI AML15,16,17 trials in patients satisfying the eligibility criteria of QUANTUM-R.
Methods: Patients aged 18+ in the UK NCRI AML15,16,17 trials were identified who harboured a FLT3 ITD mutation, were treated with intensive chemotherapy, and were either refractory to two courses of induction therapy, or relapsed within six months of transplant, or did not receive a prior transplant and had a remission duration of 6 months or less. Patients were grouped hierarchically as refractory, relapsed post transplant, or relapsed without prior transplant. Eligibility was established at the point a patient first became eligible for analysis. The primary outcome was overall survival (OS), measured from point of eligibility, with subsequent remission with or without count recovery as secondary outcome. A sensitivity analysis was performed excluding those who died within 21 days of eligibility to eliminate patients who might be thought of as too unwell to enter a post-relapse trial. Cox regression was used to identify prognostic factors for survival.
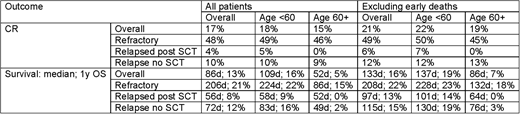
Results: A total of 264 patients were identified (refractory n=58, relapsed post SCT n=49, relapsed without SCT n=157). The median age was 51 (range 18-84); 25% of patients were aged 60 or older; 44% were male, 95% had intermediate cytogenetics; 11% had secondary disease. Split by age, among those under 60 45 were refractory, 44 relapsed post SCT and 110 relapsed without SCT; for ages 60+ the figures were 13 vs 5 vs 47. Overall 17% of patients experienced a subsequent remission; with median survival of 86 days and 1 year OS of 13%. If deaths within 21 days were excluded, the remission rate improved to 21%; with a median survival of 133 days and 1 year OS of 16%.
In multivariable Cox regression, age group HR for age>60 1.81 (1.33-2.47) p=0.0002) and route to eligibility (HR refractory vs relapsed no SCT 0.77 (0.55-1.07); relapsed post SCT vs no SCT 1.58 (1.11-2.25) p=0.003) were the only factors affecting survival - in particular sex, secondary disease, and ITD allelic burden were not significant. In the sensitivity analyses, only age was significant (HR 1.77 (1.24-2.53) p=0.001); with route to eligibility not significant (p=0.14).
Among patients with post-relapse treatment information, 65% were treated intensively, 8% non-intensively, and 20% with palliation - other patients received experimental therapies. When restricting attention to those treated intensively, median survival was 130 days with 17% 1 year OS. Figures were not materially changed if early death was excluded.
Of 215 patients who had not relapsed post transplant, 53 (25%) received a transplant post-eligibility. In these 56 patients, median survival was 301 days with 42% alive at one year.
Conclusions: In relapsed/refractory AML, outcomes for FLT3-ITD mutated patients are generally poor and worse for older patients. Applying the eligibility criteria of QUANTUM-R and excluding early deaths gives outcomes comparable to the control group of the QUANTUM-R study. In the 25% of patients who proceeded to transplant survival was extended indicating that a treatment which can deliver patients to transplant has the potential to improve patient outcomes.
References: [1] Ravandi F, et al. Leuk Res. 2010;34(6):752-756; [2] Cortes et al. EHA 2018, Abstr LB2600.
Hills:Daiichi Sankyo: Consultancy, Honoraria. Russell:Daiichi Sankyo: Consultancy; Pfizer: Consultancy, Honoraria, Speakers Bureau; Jazz Pharma: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal