Abstract
[Introduction]
Infant AML has been defined as AML in patients aged <1 year. Infant AML is related to high frequencies of M4, M5, and M7 subtypes in the FAB classification. Molecular analyses showed that KMT2A rearrangement (KMT2A-R), CBFA2T3-GLIS2 and NUP98-KDM5A are the most frequent abnormalities in infant AML. These findings indicate a distinct biology of infant AML. However, a more clinically adequate definition of "infant AML" by age remains controversial; a recent report by the COG defined patients aged <3 years with AML as infants because of their molecular similarities. Moreover, although Infants with AML are generally treated with dose-reduced regimens to avoid severe toxicities, it remains unknown whether patients aged 1-<3 years also require the same dose-reduced regimens as infants. Thus, further identification of AML in young pediatric patients is necessary.
[Methods]
This study enrolled patients aged <18 years who were diagnosed with de novo AML and who had participated in one of the two recent clinical trials in Japan: the AML99 trial and the AML-05 trial conducted by the Japanese Pediatric Leukemia/Lymphoma Study Group. In total, 723 patients were analyzed in the current study. Among them, bone marrow samples of 503 patients (70%) were screened for fusion genes and gene mutations.
[Results]
We first analyzed the distribution of fusion genes and FAB classification in 503 patients with clinical samples. Fusion genes were found in >50% of the patients in each age group. RUNX1-RUNX1T1, KMT2A-R, and CBFB-MYH11 were present in almost all age groups. RUNX1-RUNX1T1 was the most frequently identified fusion gene in all age groups excluding those <3 years, whereas KMT2A-R was the most frequently identified fusion gene in patients aged <3 years. Regarding other fusions, CBFA2T3-GLIS2 and NUP98-KDM5A were essentially limited to patients aged <3 years, whereas CBFB-MYH11 was detected in nearly all age groups. In the assessment of FAB classification, patients with M4 and M5 accounted for 50% of patients aged <3 years. Among patients aged <3 years, M7 was the second most common morphology. In total, approximately 80% of patients aged <3 years were classified as M4, M5, or M7. Conversely, M1 and M2, both of which display granulocytic differentiation, were the most frequent phenotypes among patients aged 3-<18 years. According to the distribution of fusion genes and FAB classification, we divided pediatric AML patients into two age groups: patients aged <3 years (patients <3y) and those aged 3-<18 years (patients 3-<18y). Further, we compared the prognostic effect of molecular characteristics between these two age groups, and identified KMT2A-R and CBFB-MYH11 as age-specific prognostic markers.
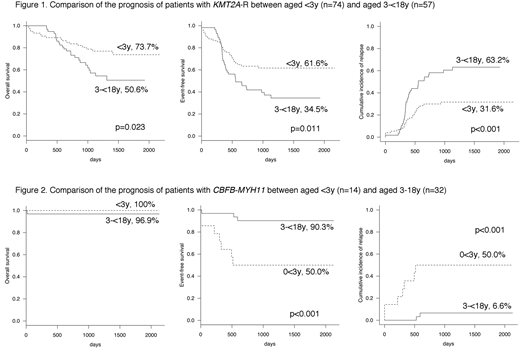
Furthermore, we analyzed the prognosis of KMT2A-R and CBFB-MYH11 in all 723 patients and confirmed that these fusions had significantly different prognoses between patients<3y and those 3-<18y. In the subgroup of patients with KMT2A-R (n = 131), 5-year (5y) OS, EFS, and CIR were significantly worse in patients 3-<18y (n = 57) than in patients <3y (n = 74) (50.6% vs 73.7%, p = 0.023; 34.5% vs 61.6%, p = 0.011; and 63.2% vs 31.6%, p <0.001, respectively) (Figure 1). Conversely, in the subgroup of patients with CBFB-MYH11 (n = 46), 5y EFS and CIR were significantly worse in patients <3y (n = 14) than in patients 3-<18y (n = 32) (50.0% vs 90.3%, p<0.001 and 50.0% vs 6.6%, p <0.001, respectively) (Figure 2).
In patients with KMT2A-R (n = 131), no molecular markers were identified as prognostic markers in combination with age; however, high WBC counts were related to poor prognosis regardless of age. Particularly, among patients 3-<18y (n = 57), those with WBC counts >10,000/µL (n = 37) had a significantly poorer prognosis than those with WBC <10,000/µL (n = 20) (5y OS 31.0% vs 78.8%, p = 0.002; 5y EFS 22.1% vs 54.2%, p = 0.008; and 5y CIR 77.9% vs 40.4%, p = 0.005).
In patients with CBFB-MYH11 with clinical samples (n = 39), NRAS-mutated patients (n = 13) had significantly better 5y EFS and CIR than NRAS-wild-type patients (n = 26) (100% vs 60.5%, p = 0.012, and 0% vs 35.7%, respectively). Moreover, the combination of NRAS status with age identified the worst prognosis in NRAS-wild-type patients <3y (5y EFS 22.2% and 5y CIR 77.8%).
[Conclusions]
Although age has not been used for the risk stratification of recent pediatric AML clinical trials, it might be a useful prognostic maker of this disease in combination with some molecular makers.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal