Abstract
Introduction: The use of lenalidomide (len) in the treatment of newly diagnosed multiple myeloma (NDMM) as induction and/or maintenance therapy is increasing. The majority of patients (pts) progress and require further treatment, highlighting a need for effective regimens for these len-exposed and len-refractory RRMM pts. Daratumumab (DARA) is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor and immunomodulatory mechanism of action. In three phase 3 studies, the addition of DARA to standard of care (SOC) regimens has doubled complete response (CR) rates, tripled minimal residual disease (MRD)-negative rates, and reduced the risk of progression or death by ≥50% vs SOC alone in RRMM and NDMM pts (Palumbo A, et al. N Engl J Med 2016. 375[8]:754-766; Dimopoulos MA, et al. N Engl J Med 2016. 375[14]:1319-1331; Mateos MV, et al. N Engl J Med 2018. 378[6]:518-528). To evaluate the efficacy of DARA plus SOC regimens in RRMM pts previously exposed or refractory to len, we evaluated data from relevant subpopulations of patients in the phase 3 CASTOR and POLLUX studies and the phase 1 MMY1001 study.
Methods: CASTOR and POLLUX are both open-label, randomized, phase 3 studies of DARA plus bortezomib/dexamethasone (D-Vd) or lenalidomide/dexamethasone (D-Rd), respectively, vs SOC alone in RRMM pts with ≥1 prior line of therapy. Len-refractory pts were ineligible for POLLUX. Within MMY1001, a multi-arm phase 1b study, RRMM pts treated with DARA plus carfilzomib/dexamethasone (D-Kd) or pomalidomide/dexamethasone (D-Pd) were included in this analysis. In the phase 3 studies, progression-free survival (PFS) was assessed in the intent-to-treat (ITT) population and were compared using a stratified log-rank test. Responses were assessed in an evaluable population defined as pts with measurable disease at baseline and ≥1 post-baseline disease assessment. Minimal residual disease (MRD) was evaluated in the ITT population using clonoSEQ® V2.0 (Adaptive Biotechnologies, Seattle, WA).
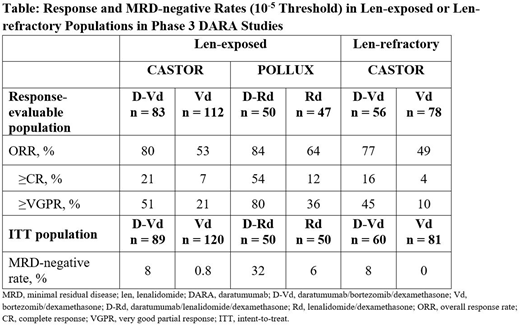
Results: Median (range) number of prior lines received was 2 (1-10) in CASTOR, 1 (1-11) in POLLUX, 2 (1-4) in the MMY1001 D-Kd cohort, and 4 (1-13) in the MMY1001 D-Pd cohort. A total of 493 pts (323 pts treated with DARA) received prior len across the 3 DARA studies. Among len-exposed pts in CASTOR (D-Vd, n = 89; Vd, n = 120), median PFS was 9.5 vs 6.1 months (hazard ratio [HR] 0.40; 95% confidence interval [CI], 0.28-0.58; P <0.0001) after median follow up of 31.3 months. A similar PFS HR was reported for len-exposed pts in POLLUX (D-Rd, n = 50; Rd, n = 50) after median follow up of 39.5 months, in which D-Rd treated pts demonstrated significantly longer PFS vs Rd treated pts (median: 38.9 mo vs 18.6 mo; HR 0.39; 95% CI, 0.22-0.70; P = 0.0010). In both the CASTOR and POLLUX studies, rates of deeper responses and MRD-negative rates at 10-5 sensitivity threshold were all significantly higher (P <0.05 for all comparisons) for DARA-containing regimens vs SOC alone (Table).
A total of 284 pts (203 pts treated with DARA) were len-refractory across CASTOR and MMY1001. Among 60 D-Vd and 81 Vd len-refractory pts in CASTOR, median PFS was 7.8 vs 4.9 months (HR 0.44; 95% CI, 0.28-0.68; P = 0.0002). Significantly higher response and MRD-negative rates at 10-5 were observed for D-Vd vs Vd in len-refractory pts (Table).
In MMY1001, nearly all pts treated with D-Kd (n = 81/85) or D-Pd (n = 103/103) were exposed to len, and the majority of pts treated with D-Kd (n = 51/85) or D-Pd (n = 92/103) were len-refractory. Among all pts, median PFS was not reached for D-Kd after median follow-up of 12 months (12-mo PFS rate: 62%), and median PFS was 9.9 months for D-Pd after median follow-up of 28.1 months. Among the 51 len-refractory pts treated with D-Kd, median PFS was 14.1 months. In all-treated pts, ORR was 66% for D-Pd and 84% for D-Kd (79% for the len-refractory subgroup).
Updated data from all 3 studies will be presented at the meeting.
Conclusion: In len-exposed or -refractory patients, DARA enabled deep responses and prolonged PFS irrespective of the SOC combination partner and number of prior lines of treatment. DARA-based regimens were more effective in less heavily pretreated len-exposed or len-refractory pts, suggesting earlier use (eg, after first relapse) would provide a greater benefit.
Usmani:Abbvie, Amgen, Celgene, Genmab, Merck, MundiPharma, Janssen, Seattle Genetics: Consultancy; Amgen, BMS, Celgene, Janssen, Merck, Pharmacyclics,Sanofi, Seattle Genetics, Takeda: Research Funding. Mateos:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lentzsch:Caelum Biosciences: Consultancy, Other: Dr. Lentzsch recused herself as an investigator from the Phase 1a/b trial testing CAEL-101 in 11/2017., Patents & Royalties: Shareholder for Caelum Biosiences; BMS: Consultancy; Janssen: Consultancy; Bayer: Consultancy. Quach:Celgene: Consultancy, Research Funding; Janssen Cilag: Consultancy; Sanofi Genzyme: Research Funding; Amgen: Consultancy, Research Funding. Capra:Janssen: Research Funding, Speakers Bureau; Roche: Speakers Bureau; Amgen: Speakers Bureau; AbbVie: Research Funding; Sanofi: Research Funding; Bristol Myers Squibb: Research Funding; Novartis: Research Funding. Sonneveld:Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Qi:Janssen Research & Development, LLC: Employment. Amin:Janssen Research & Development, LLC: Employment. Wang:Janssen Research & Development, LLC: Employment. Qin:Janssen Research & Development, LLC: Employment. Okonkwo:Janssen Research & Development, LLC: Employment. Ukropec:Janssen Scientific Affairs, LLC: Employment. Trivedi:Janssen Research & Development, LLC: Employment. Suzuki:Takeda: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Ono: Consultancy, Honoraria; Sanofi Aventis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; SRL.Inc: Employment. Dimopoulos:Bristol-Myers Squibb: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Celgene: Honoraria. Cavo:GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Nooka:Adaptive technologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Spectrum Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Chari:Array Biopharma: Research Funding; The Binding Site: Consultancy; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy; Pharmacyclics: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Facon:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal