Abstract
Background:
MDS originates from somatic mutations leading to clonally restricted hematopoiesis causing cytopenias and cellular dysplasia. Clonal expansion of these mutations in the absence of MDS criteria is known as clonal hematopoiesis of indeterminate potential (CHIP) and is considered a MDS precursor. CHIP is associated with increased risk of CVD, especially early onset myocardial infarction (MI) (Jaiswal S et al, NEJM, 2017). CVD events increase with clone size and a potential causal relationship involves accelerated atherosclerosis from an altered cell-mediated inflammatory response in the atherosclerotic plaque. It is suggested that MDS patients carry an increased risk of CVD mortality (Brunner AM et al, Bld Advances, 2017); it is unclear however if the increased risk of CVD persists even after developing MDS or is limited to CHIP. The influence of demographic factors & comorbidities on CVD among MDS patients is also unknown. The SEER Medicare tumor registry was queried to help answer these questions.
Methods:
Individuals 65 years or older from the SEER Medicare database with incident diagnosis of MDS between Jan. 1, 2004 and Dec. 31, 2014 (n=13,193) were identified. Demographic data and Charlson comorbidity index (CCI) 1 year prior to MDS diagnosis were analyzed. A control set of 5% randomly sampled non-cancer Medicare beneficiaries was paired to cases by propensity score matching (PSM) accounting for age, gender, race and urban/rural residence. The MDS diagnosis date served as the pseudodiagnosis date for non-cancer paired subjects. New onset MI or ischemic strokes (CVA) were identified with ICD9 and HCPCS codes in Medicare claims from date of diagnosis to death or study end. Incident cases were those with claims occurring after, and absent 1 year before, the MDS diagnosis date. Cumulative incidence functions were used to assess the risk of new events. Cox proportional hazards models, extended for time dependent covariates and coefficients, were used to evaluate clinical variables associated with new onset CVD and death.
Results:
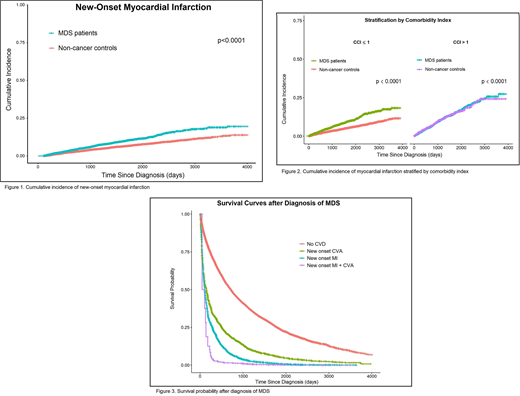
MDS patients were predominantly male (55.2%) and non-hispanic whites (82.1%) from metropolitan areas (82.5%). Median age at diagnosis was 82 years (66 - 112) and median CCI score was 0 (0 - 13). The 5-year cumulative incidence of MI was 12.5% for MDS patients vs. 7% for controls (p<0.0001; Fig. 1). A diagnosis of MDS increases the risk of new onset MI by 1.4 (95% CI, 1.2 - 1.5). Other factors that significantly influenced the hazard ratio of MI were male sex (HR 1.3, 95% CI, 1.1 - 1.4), older age at diagnosis (HR 1.04 for each year over 65, 95% CI 1.03 - 1.05) and higher CCI (HR 1.2 for each point over 0, 95% CI 1.2 - 1.3). The effect of having MDS persisted after accounting for these factors in a multivariate Cox model and was more pronounced in patients with lower CCI (50% increase in 5-year MI rate in subgroup with CCI ≤1, Fig. 2). There was no difference in cumulative incidence of CVA between groups and MDS did not impact the risk of CVA. In multivariate analysis adjusted for age, gender and CCI, MDS was an independent risk factor for mortality in patients with new onset MI (HR 2.5, 95% CI, 1.1 - 3) and new onset CVA (HR 1.7, 95% CI, 1.5 - 1.9). Similarly, both MI (HR 2.8, 95% CI, 2.6 - 3.1) and CVA (HR 1.6, 95%CI 1.4 - 1.7) were independent risk factors for mortality in MDS patients (Fig. 3).
Conclusion:
Cumulative incidence of MI was higher in Medicare beneficiaries with MDS compared to non-cancer controls. The association of MI and MDS was even stronger in patients with a lower CCI score, particularly in patients with score ≤ 1. Thus, a diagnosis of MDS confers an independent risk for early MI in relatively healthy patients with little or no prior cardiovascular comorbidities. Furthermore, survival after a new CVD event was significantly reduced in subjects with MDS compared to controls. Evaluation and modification of cardiovascular risk factors is essential in patients with MDS and should be instituted early as it directly impacts their survival. Although we used validated methods, study limitations include relying on administrative claims from Medicare beneficiaries, absence of genetic or lifestyle information and unmeasured confounding factors not accounted for by PSM. While the risk of CVA was not impacted by a diagnosis of MDS, thrombocytopenia, anemia and transfusion dependence likely influenced the risk of these ischemic events and will be further explored as cofactors.
Janakiram:Seatle Genetics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal