Abstract
Introduction
Myelodysplastic syndromes (MDS) are heterogeneous myeloid disorders characterized by dysplasia in one or more hematopoietic cell lines, peripheral cytopenia(s), and distinct cytogenetic abnormalities. The presence of certain cytogenetic abnormalities [e.g. 7/del(7q), -5/del(5q), 13/del(13q), i(17p)], in the setting of unexplained persistent cytopenia, has been considered presumptive evidence of MDS, despite definitive morphologic features of MDS. Conversely, chromosomal abnormalities such as isolated 20q- are not considered definitive evidence for MDS, in the absence of dysplasia. Based on our previous findings, patients with isolated 20q- without morphologic features of a myeloid neoplasm have varied clinical outcomes (Jawad MD et al. Am J Hematol.2016). In this study, we investigate the frequency of recurrent somatic gene mutations in these patients, and their relationship to progression to a myeloid neoplasm.
Methods
We retrospectively reviewed the bone marrow pathology reports of patients evaluated at our institution from 01/2005 to 09/2015 who had isolated 20q- in at least 2 of 20 metaphases. Patients with isolated 20q- and without myeloid neoplasms were included in the study, along with medical record review. A 35-gene next generation sequencing (NGS) panel (OncoHeme) targeting myeloid neoplasms was performed on available archived bone marrow aspirate pellets. Statistical analyses were performed using JMP Pro software version 13. Parametric (t-test) or nonparametric tests such as the Fisher-exact test, chi-square test, or Wilcoxon rank-sum test, were utilized as appropriate for test variables. Statistical significance was based on a 2-sided significance level of .05. Kaplan-Meier analysis was used for analyzing time to progression to myeloid neoplasms and progression-free survival (i.e. time to progression to myeloid neoplasms or death from any cause).
Results
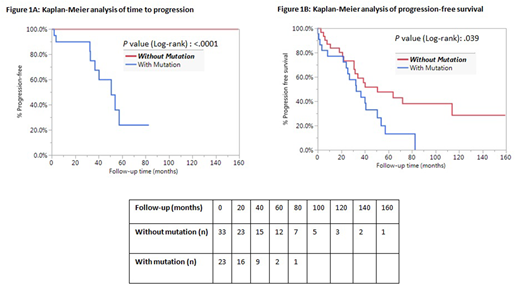
Fifty-six (42 males, 14 females) patients with isolated 20q- were identified. The average percentage of 20q- was 38.5 % (±29.3). The median age was 68 years (range: 44-90) and the median hemoglobin (Hb), absolute neutrophil count (ANC) and platelet count (Plt) were 11.7 g/dL (range: 8.3-15.1), 2.7 x 10(9)/L (range: 0.6-9.4) and 138 x 10(9)/L (range: 16-392), respectively. The indications for bone marrow biopsies ranged from cytopenia (21), multiple myeloma (20), non-Hodgkin lymphoma (10), amyloidosis (2), CLL (2) and transplant donor evaluation (1). Twenty-three patients harbored pathogenic mutations (15 had one mutation, 1 had two mutations and 7 had three mutations). The identified pathogenic mutations included ASXL1 (8), TET2 (8), SRSF2(3),PHF6 (2), SF3B1 (2), CBL (2), U2AF1 (2), IDH1 (1), IDH2 (1), BCOR (1), JAK2 (1), PTPN11(1), TP53 (1) and RUNX1 (1). Nine patients (16.1%) progressed to myeloid neoplasms (5 MDS, 2 acute myeloid leukemia, 2 chronic myeloid neoplasm-not otherwise specified) at a median follow-up of 36.7 months (range: 1.9-57.4). Of these 9 patients, 5 had one mutation (BCOR, TET2, DNM3TA, CBL, IDH2), 1 had two mutations (SF3B1/CBL) and 3 had three mutations (ASXL1/IDH1/SRSF2,DNM3TA/PTPN11/TET2, PHF6/TET2/TET2). In contrast, only 29.8% of non-progressed cases showed at least 1 mutation. Kaplan-Meier analysis demonstrated the presence of genetic mutations in patients with isolated 20q- carried significantly higher chance of progression to myeloid neoplasms (p<.05, Figure 1A and 1B). However, the number of genetic mutations was not predictive of progression in our relatively small study cohort (p=.66). There was no significant difference between those who did and did not progress with respect to age, gender, Hb, ANC, Plt, 20q- or mutational allele burden.
Conclusions
Most of the patients with isolated 20q- and without morphologic features of a myeloid neoplasm did not harbor myeloid neoplasm associated gene mutations and further did not develop a myeloid neoplasm during the course of our study. Among patients with pathogenic mutations, nearly one-third showed progression to a myeloid neoplasm. Of the clinical and laboratory variables, the presence of genetic mutation is a significant risk factor for progression to a myeloid neoplasm. This study revealed the underlying molecular heterogeneity of isolated 20q- in the absence of morphologic features of a myeloid neoplasm, and underscored the prognostication value of genetic mutation analysis in these cases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal