Abstract
Introduction: Published guidelines recommend mobilization of allogeneic peripheral blood progenitor cells (PBPC) with 4-5 days of granulocyte colony-stimulating factor (G-CSF) followed by PBPC collection on day 5. Given that some autologous transplant patients are adequately mobilized by day 4, and concern that a subset of allogeneic donors may be maximally mobilized before day 5 of G-CSF administration, we performed a feasibility study evaluating day 4 allogeneic PBPC collection.
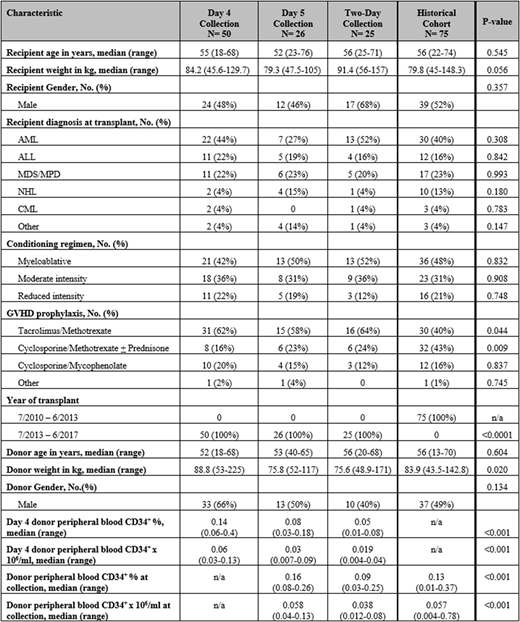
Methods: This single-center prospective study included adult patients with malignant diseases who received a first allogeneic hematopoietic stem cell transplant (HCT) at our center between 7/1/2013 - 6/30/2017. HLA-matched sibling donors received 10 μg/kg of G-CSF subcutaneously prior to PBPC collection. Donors were collected on day 4 of G-CSF if peripheral blood (PB) CD34+ counts were ≥0.04x106/ml. Donors with day 4 PB CD34+ counts <0.04x106/ml were collected on day 5, and those with inadequate collected CD34+ cells/kg recipient weight underwent repeat collection over two days. Patient and donor characteristics, PBPC product composition, transplant outcomes, and cost analysis of the PBPC collection procedure were compared to a historical cohort of donors collected on day 5 according to our prior algorithm.
Results: One hundred and one patients/donors were eligible for inclusion on study, and 75 were included in the historical cohort. Of the 101 donors on study, 50 (49.5%) had adequate PBPC collection on day 4 of G-CSF, 26 (25.7%) were collected on day 5, and 25 (24.8%) required two-day collections on days 4 and 5. The median day 4 donor PB CD34+ cell count was 0.06 (range 0.03-0.13) x 106/ml among donors collecting on day 4, 0.03 (range 0.007-0.09) x 106/ml for donors collecting on day 5, and 0.019 (range 0.004-0.04) x 106/ml for donors collecting over two-days (p<0.001).
The most common reported donor symptoms were bone pain, myalgia/malaise, headache, and circumoral tingling/electrolyte abnormalities. Compared to the historical cohort, donors with PBPC collection on day 4 were significantly more likely to develop bone pain, require narcotics, and take over-the-counter (OTC) medications, day 5 donors were significantly more likely to develop bone pain and take OTC medications, and two-day donors were significantly more likely to develop bone pain and circumoral tingling/electrolyte abnormalities.
The median collected PBPC CD34+ cell count (7.25 x 106/kg recipient weight) and infused PBPC product CD34+ cell count (5.67 x 106/kg recipient weight) were significantly greater in products collected on day 4, compared to products collected on other days. In comparison, the infused PBPC product total nucleated cell (TNC) count (7.13 x 108/kg recipient weight), mononuclear cell (MNC) count (5.84 x 108/kg recipient weight), and CD3+ cell count (2.14 x 108/kg recipient weight) were significantly lower in products collected on day 4 compared to other collection cohorts (p<0.001, p<0.001, and p=0.002, respectively for day 5, two-day, and historical cohort).
There were no significant differences in time to neutrophil or platelet engraftment, or in the incidence of acute or chronic graft-versus-host-disease, based on day of collection. There was a non-significant trend towards improved 1-year survival among patients transplanted with PBPC collected on day 4 (p=0.077). Cost analysis revealed a 6.7% savings in direct costs per collection procedure for the day 4 collection method versus historical cohort.
Conclusions: Using a day 4 PB CD34+ threshold of ≥0.04x106/ml identifies donors with a high likelihood of adequate PBPC collection. While differences in PBPC product composition and donor symptoms were seen based on day of collection, we found no evidence of adverse post-HCT outcomes associated with day 4 PBPC collection. Day 4 may be the optimal day of stem cell collection for a population of healthy donors without adverse effect on transplant outcomes, and with an anticipated cost savings. Ongoing analyses include resource utilization review, matched cohort analysis of day 4 versus 5 collection and correlation with transplant outcomes, more detailed PBPC production composition analysis among the collection cohorts, and longer post-HCT follow-up and association with outcomes based on day of PBPC collection. These findings merit further analysis in larger HCT cohorts.
Cook:Syros Pharmaceuticals: Consultancy. Maziarz:Incyte: Consultancy, Honoraria; Kite Therapeutics: Honoraria; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Athersys, Inc.: Patents & Royalties; Juno Therapeutics: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal