Key Points
HCT-CI scores ≥3 are associated with inferior survival after allogeneic transplantation for nonmalignant diseases.
The HCT-CI does not predict risk of mortality from transplantation for patients with hemoglobinopathies.
Abstract
Despite improvements, mortality after allogeneic hematopoietic cell transplantation (HCT) for nonmalignant diseases remains a significant problem. We evaluated whether pre-HCT conditions defined by the HCT Comorbidity Index (HCT-CI) predict probability of posttransplant survival. Using the Center for International Blood and Marrow Transplant Research database, we identified 4083 patients with nonmalignant diseases transplanted between 2007 and 2014. Primary outcome was overall survival (OS) using the Kaplan-Meier method. Hazard ratios (HRs) were estimated by multivariable Cox regression models. Increasing HCT-CI scores translated to decreased 2-year OS of 82.7%, 80.3%, 74%, and 55.8% for patients with HCT-CI scores of 0, 1 to 2, 3 to 4, and ≥5, respectively, regardless of conditioning intensity. HCT-CI scores of 1 to 2 did not differ relative to scores of 0 (HR, 1.12 [95% CI, 0.93-1.34]), but HCT-CI of 3 to 4 and ≥5 posed significantly greater risks of mortality (HR, 1.33 [95% CI, 1.09-1.63]; and HR, 2.31 [95% CI, 1.79-2.96], respectively). The effect of HCT-CI differed by disease indication. Patients with acquired aplastic anemia, primary immune deficiencies, and congenital bone marrow failure syndromes with scores ≥3 had increased risk of death after HCT. However, higher HCT-CI scores among hemoglobinopathy patients did not increase mortality risk. In conclusion, this is the largest study to date reporting on patients with nonmalignant diseases demonstrating HCT-CI scores ≥3 that had inferior survival after HCT, except for patients with hemoglobinopathies. Our findings suggest that using the HCT-CI score, in addition to disease-specific factors, could be useful when developing treatment plans for nonmalignant diseases.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a curative therapy for many nonmalignant diseases, including congenital or acquired bone marrow failure syndromes, primary immune deficiency or dysregulation disorders, hemoglobinopathies, and metabolic diseases.1-9 Successful HCT for nonmalignant diseases can be curative, but transplant-related mortality remains a concern.1,2,9 There is a need for better understanding of the risks associated with mortality after HCT in these patients. Although efforts have evaluated the effects of primary disease and transplant characteristics, limited investigation has been performed on the role of patient-specific factors in this population.1-9
Comorbidities influence treatment outcomes of several primary diseases.10-14 In the field of HCT, the HCT Comorbidity Index (HCT-CI), a composite score of 17 weighted comorbidities, has been validated and shown to discriminate risk of nonrelapse and overall mortality following HCT.15 However, studies have primarily focused on patients with hematologic malignancies and included few patients with nonmalignant diseases.15-26 Patients with nonmalignant diseases differ from those with hematologic malignancies in their prior treatment exposures and profile of acquired and disease-related comorbidities.6,16,27-29 Further, assessment of “nonrelapse mortality” is difficult to assess in patients with nonmalignant diseases, in which even low-level donor hematopoiesis can be curative for many patients.30,31 Relapse of underlying disease is a competing risk for death after HCT, which is often difficult to define and may not be relevant in patients who undergo HCT for nonmalignant diseases. This raises the question of whether the HCT-CI can predict the unique outcomes of patients with nonmalignant diseases after allogeneic HCT.
To this end, we: (1) studied the profile of pre-HCT comorbidities, per the HCT-CI, in patients with nonmalignant diseases; (2) evaluated the association between HCT-CI scores and survival in patients undergoing HCT for nonmalignant diseases; and (3) identified performance of the HCT-CI by type of nonmalignant disease and conditioning intensity.
Methods
This study used data available from the Center for International Blood and Marrow Transplant Research (CIBMTR), a research affiliate of the International Bone Marrow Transplant Registry and National Marrow Donor Program. More than 450 transplant centers worldwide report baseline characteristics and longitudinal outcome data to the CIBMTR. Compliance is monitored by onsite audits. Observational studies performed by the CIBMTR are performed in compliance with all applicable US federal regulations pertaining to the protection of human research participants. Protected health information is collected and maintained in the CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule.
A prospective, observational study was developed by our team in conjunction with the CIBMTR in 2007 to collect information on pre-HCT comorbidities for all patients receiving allogeneic HCT in order to assess the predictive power of the HCT-CI.16 HCT-CI–defining comorbidities were collected on the Pre-Transplant Essential Data (pre-TED) form.15 An education session for all data managers was held to describe accurate comorbidity coding for the HCT-CI and was made public on the CIBMTR Web site and in the Forms Instruction Manual.32 Subsequently, an online HCT-CI calculator was made available to aid in HCT-CI calculation.33
Inclusion and exclusion criteria
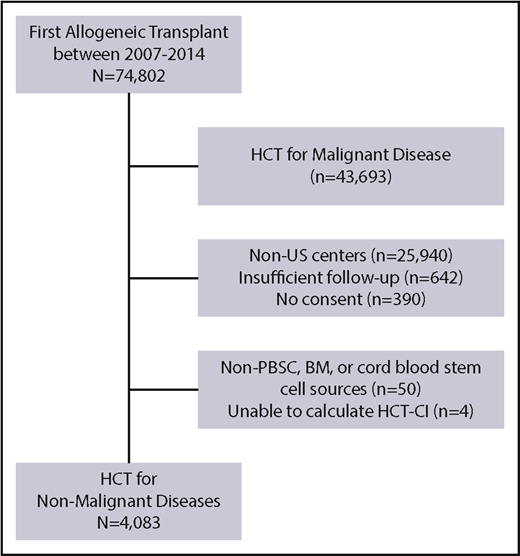
HCT-CI scores were calculated on patients who underwent first allogeneic HCT for a nonmalignant indication between 1 December 2007 and 31 December 2014. Figure 1 demonstrates patient eligibility and inclusion criteria. Patients were limited to those with nonmalignant diseases from US centers with follow-up of >100 days post-HCT and from centers reporting >70% follow-up data to ensure completeness of data. Of the 74 822 recipients of first HCT who were reported to the CIBMTR during this period, 4083 fulfilled eligibility and were included in this analysis. Follow-up forms were reported to the CIBMTR at 100 days, 6 months, and yearly after HCT for each patient.
Criteria for inclusion and exclusion. BM, bone marrow; PBSC, peripheral blood stem cell.
Criteria for inclusion and exclusion. BM, bone marrow; PBSC, peripheral blood stem cell.
Study outcomes and definitions
The primary outcome of analysis was overall survival (OS). The variables assessed in the analysis are described in the following sections.
Age groups.
HCT-CI score.
HCT-CI scores were calculated by CIBMTR statisticians after review of comorbidities filed on the pre-TED forms.15 Missing entries for comorbidities were present in <1% of patients. HCT-CI scores were grouped as 0, 1 to 2, 3 to 4, and ≥5 in analysis of the entire cohort and grouped as 0, 1 to 2, and ≥3 for subgroup analyses (because of limited patient numbers).16
Disease categorization.
Patients were categorized based on their primary bone marrow, metabolic, or immune defects into the following categories: acquired aplastic anemia, hemoglobinopathies, primary immune deficiencies, congenital bone marrow failure syndromes, histiocytic disorders, metabolic diseases, and autoimmune diseases (supplemental Table 1, available on the Blood Web site).
Conditioning intensity.
Details of conditioning regimens for each patient were collected on CIBMTR forms. High-dose myeloablative conditioning was defined based on chemotherapy and radiation dosing, as previously described (details in supplemental Table 2).34 Immunosuppressive conditioning was defined as nonmyeloablative regimens containing fludarabine or low-dose cyclophosphamide with or without antithymocyte globulin (ATG) or alemtuzumab.5,35 Sixty-five patients with severe combined immune deficiency (SCID) did not receive conditioning before HCT infusion. The remaining regimens were grouped as reduced-intensity regimens.34
Graft-versus-host disease prophylaxis.
Patients who received tacrolimus or cyclosporine as their primary GVHD prophylaxis agent were grouped under calcineurin inhibitor–based regimens (alone or with methotrexate, mycophenolate mofetil, or others). Patients who received ex vivo T-cell depletion or CD34 selection were grouped under “ex vivo T-cell depletion.” Additionally, serotherapy with ATG or alemtuzumab was also assessed as an independent variable defined as “in vivo T-cell depletion.” A subset of patients was not given any GVHD prophylaxis and hence were grouped under “None” (supplemental Table 3).
Statistical analysis
Patient demographics, disease indications, HCT characteristics, and comorbidities were described using frequencies/percentages or median (range) as appropriate. Survival rates were calculated using Kaplan-Meier estimates stratified by HCT-CI scores and compared using the log-rank test. Patients were censored at time of last follow-up.
Multivariable Cox regression analysis was performed to assess the effect of the HCT-CI on OS while adjusting for potential confounders.36 A HCT-CI score of 0 was set as the reference value. The HCT-CI score was forced into the model; stepwise multivariable selection was used to identify additional variables to include in the model. Variables considered included age, sex, race, disease category, donor and HLA-match, graft source, recipient cytomegalovirus (CMV) status, in vivo T-cell depletion, transplant year, conditioning intensity, GVHD prophylaxis, and performance status. Proportional hazards assumption was assessed for all variables using graphical methods. Interactions between HCT-CI and other variables were assessed, but none was identified as significant. Subgroup analyses were conducted based on conditioning intensity and disease categorization using the same variables as outlined previously in the final regression model, except for the metabolic and autoimmune disease groups because of limited patient or event numbers. All p values are 2-sided and significance was defined as P < .05. SAS 9.3 (SAS Inc., Cary, NC) was used for all analyses.
Results
Patient characteristics
Patient, disease, and HCT characteristics are noted in Table 1. The median age was 9 years (range, <1 to 77) with 68% of patients <20 years old. The most common indications for HCT were acquired aplastic anemia (33%), primary immune deficiency (19%), and hemoglobinopathies (16%).
Patient and transplant characteristics of patients with nonmalignant diseases
| Variable . | N (%) . |
|---|---|
| No. of patients | 4083 |
| Age, y | |
| 0-2 | 908 (22) |
| 2-10 | 1322 (32) |
| 10-20 | 965 (24) |
| >20 | 888 (22) |
| Median age (range) | 9 (<1-77) |
| Recipient sex | |
| Male | 2417 (59) |
| Female | 1666 (41) |
| Race | |
| White | 2811 (69) |
| African American | 791 (19) |
| Asian | 255 (6) |
| Pacific islander | 14 (<1) |
| Native American | 51 (1) |
| Missing | 161 (4) |
| Recipient CMV status | |
| Negative | 1708 (42) |
| Positive | 2291 (56) |
| Missing | 84 (2) |
| Performance status (Lansky <18 y, Karnofsky >18 y) | |
| 90-100 | 3210 (79) |
| <90 | 776 (19) |
| Missing | 97 (2) |
| HCT-CI | |
| 0 | 2512 (62) |
| 1-2 | 821 (20) |
| 3-4 | 556 (14) |
| >5 | 194 (5) |
| Regroup disease categorization | |
| Aplastic anemia | 1337 (33) |
| BM failure | 470 (12) |
| Hemoglobinopathies | 656 (16) |
| Immune deficiency | 796 (19) |
| Metabolic disease | 371 (9) |
| Histiocytic disorders | 432 (11) |
| Autoimmune disease | 21 (<1) |
| Transplant-related variables | |
| Donor and graft | |
| 8/8 Matched related donor BM | 1118 (27) |
| 8/8 Matched related donor PBSC | 133 (3) |
| ≤7/8 Related donor BM | 135 (3) |
| ≤7/8 Related donor PBSC | 118 (3) |
| 8/8 Unrelated donor BM | 922 (23) |
| 8/8 Unrelated donor PBSC | 225 (6) |
| 7/8 Unrelated donor BM | 282 (7) |
| 7/8 Unrelated donor PBSC | 90 (2) |
| ≤6/8 Unrelated donor BM or PBSC | 102 (2) |
| 6/6 Cord | 163 (4) |
| 5/6 Cord | 282 (7) |
| ≤4/6 Cord | 450 (11) |
| Missing HLA-match | 63 (2) |
| Donor sex | |
| Male | 1854 (45) |
| Female | 1330 (33) |
| Cord blood | 895 (22) |
| Missing | 4 (<1) |
| Conditioning intensity* | |
| Myeloablative | 1439 (35) |
| Bu/Cy based | 1000 |
| Bu/Flu based | 342 |
| TBI based | 51 |
| Others | 46 |
| Reduced intensity | 1820 (45) |
| Flu/Mel based | 911 |
| Flu/Cy + others | 479 |
| Bu/Flu based | 104 |
| Cy/TBI based | 154 |
| Others | 172 |
| Immunosuppressive | 759 (19) |
| Cy | 444 |
| Flu/Cy | 263 |
| Others | 52 |
| None planned | 65 (2) |
| GVHD prophylaxis† | |
| Calcineurin inhibitor ± others (not MTX, MMF) | 719 (18) |
| Calcineurin inhibitor + methotrexate based | 1725 (42) |
| Calcineurin inhibitor + MMF (not MTX) based | 1205 (30) |
| T-cell depletion ± other | 287 (7) |
| Others | 120 (3) |
| None | 25 (<1) |
| Missing | 2 (<1) |
| In vivo T-cell depletion | |
| ATG | 2240 (55) |
| Campath | 1259 (31) |
| None | 584 (14) |
| HCT year | |
| 2007 | 38 (<1) |
| 2008 | 451 (11) |
| 2009 | 573 (14) |
| 2010 | 528 (13) |
| 2011 | 612 (15) |
| 2012 | 603 (15) |
| 2013 | 669 (16) |
| 2014 | 609 (15) |
| Variable . | N (%) . |
|---|---|
| No. of patients | 4083 |
| Age, y | |
| 0-2 | 908 (22) |
| 2-10 | 1322 (32) |
| 10-20 | 965 (24) |
| >20 | 888 (22) |
| Median age (range) | 9 (<1-77) |
| Recipient sex | |
| Male | 2417 (59) |
| Female | 1666 (41) |
| Race | |
| White | 2811 (69) |
| African American | 791 (19) |
| Asian | 255 (6) |
| Pacific islander | 14 (<1) |
| Native American | 51 (1) |
| Missing | 161 (4) |
| Recipient CMV status | |
| Negative | 1708 (42) |
| Positive | 2291 (56) |
| Missing | 84 (2) |
| Performance status (Lansky <18 y, Karnofsky >18 y) | |
| 90-100 | 3210 (79) |
| <90 | 776 (19) |
| Missing | 97 (2) |
| HCT-CI | |
| 0 | 2512 (62) |
| 1-2 | 821 (20) |
| 3-4 | 556 (14) |
| >5 | 194 (5) |
| Regroup disease categorization | |
| Aplastic anemia | 1337 (33) |
| BM failure | 470 (12) |
| Hemoglobinopathies | 656 (16) |
| Immune deficiency | 796 (19) |
| Metabolic disease | 371 (9) |
| Histiocytic disorders | 432 (11) |
| Autoimmune disease | 21 (<1) |
| Transplant-related variables | |
| Donor and graft | |
| 8/8 Matched related donor BM | 1118 (27) |
| 8/8 Matched related donor PBSC | 133 (3) |
| ≤7/8 Related donor BM | 135 (3) |
| ≤7/8 Related donor PBSC | 118 (3) |
| 8/8 Unrelated donor BM | 922 (23) |
| 8/8 Unrelated donor PBSC | 225 (6) |
| 7/8 Unrelated donor BM | 282 (7) |
| 7/8 Unrelated donor PBSC | 90 (2) |
| ≤6/8 Unrelated donor BM or PBSC | 102 (2) |
| 6/6 Cord | 163 (4) |
| 5/6 Cord | 282 (7) |
| ≤4/6 Cord | 450 (11) |
| Missing HLA-match | 63 (2) |
| Donor sex | |
| Male | 1854 (45) |
| Female | 1330 (33) |
| Cord blood | 895 (22) |
| Missing | 4 (<1) |
| Conditioning intensity* | |
| Myeloablative | 1439 (35) |
| Bu/Cy based | 1000 |
| Bu/Flu based | 342 |
| TBI based | 51 |
| Others | 46 |
| Reduced intensity | 1820 (45) |
| Flu/Mel based | 911 |
| Flu/Cy + others | 479 |
| Bu/Flu based | 104 |
| Cy/TBI based | 154 |
| Others | 172 |
| Immunosuppressive | 759 (19) |
| Cy | 444 |
| Flu/Cy | 263 |
| Others | 52 |
| None planned | 65 (2) |
| GVHD prophylaxis† | |
| Calcineurin inhibitor ± others (not MTX, MMF) | 719 (18) |
| Calcineurin inhibitor + methotrexate based | 1725 (42) |
| Calcineurin inhibitor + MMF (not MTX) based | 1205 (30) |
| T-cell depletion ± other | 287 (7) |
| Others | 120 (3) |
| None | 25 (<1) |
| Missing | 2 (<1) |
| In vivo T-cell depletion | |
| ATG | 2240 (55) |
| Campath | 1259 (31) |
| None | 584 (14) |
| HCT year | |
| 2007 | 38 (<1) |
| 2008 | 451 (11) |
| 2009 | 573 (14) |
| 2010 | 528 (13) |
| 2011 | 612 (15) |
| 2012 | 603 (15) |
| 2013 | 669 (16) |
| 2014 | 609 (15) |
Bu, busulfan; CNI, calcineurin inhibitor; Cy, cyclophosphamide; Flu, fludarabine; Mel, melphalan; MMF, mycophenolate mofetil; MTX, methotrexate; TBI, total body irradiation.
See supplemental Table 1 for conditioning regimens.
See supplemental Table 2 for GVHD prophylaxis regimens.
Most patients received bone marrow as a stem cell source (63%). HLA-matched related (30%) or unrelated (29%) donors were most common, and cord blood donors were used in 22% of patients. Reduced-intensity conditioning regimens were used in 45% of patients, with fludarabine/melphalan-based regimens the most common. Thirty-five percent of patients received myeloablative conditioning, with busulfan/cyclophosphamide regimens predominating. Most patients received in vivo T-cell depletion with ATG or alemtuzumab (86%). The majority of patients had performance status scores ≥90% (79%) and HCT-CI scores of 0 (62%). All patients had follow-up data available at a median of 39 months post-HCT (range, 4-98).
Prevalence of comorbidities and distribution of HCT-CI scores
The most frequent comorbidities represented in the entire cohort of patients with nonmalignant diseases were pulmonary disease (16%), hepatic disease (13%), and infection (8%) (Figure 2). Certain comorbidities were rare (≤1%), including inflammatory bowel disease, renal disease, peptic ulcer disease, rheumatologic disease, and prior solid tumors.
Distribution of comorbidities in patients with nonmalignant diseases.
HCT-CI assessment in all patients with nonmalignant diseases
Increasing HCT-CI scores translated to lower OS, with a 2-year OS of 82.7% (95% confidence interval [CI], 81.2-84.2) for patients with HCT-CI scores of 0, 80.3% (95% CI, 77.6-83.2) with those with scores of 1 to 2, 74% (95% CI, 70.3-77.8) for those with scores of 3 to 4, and 55.8% (95% CI, 47.9-62.8) for those with scores ≥5 (Figure 3).
Kaplan-Meier estimates for overall survival in the entire cohort and subgroup analyses. (A) Entire nonmalignant cohort. (B) Aplastic anemia (n = 1337). (C) Immune deficiency (n = 796). (D) Hemoglobinopathies (n = 656). (E) Histiocytic disorders (n = 432). (F) Bone marrow failure (n = 470). (G) Immunosuppressive conditioning (n = 759). (H) Reduced-intensity conditioning (n = 1820). (I) Myeloablative conditioning (n = 1439).
Kaplan-Meier estimates for overall survival in the entire cohort and subgroup analyses. (A) Entire nonmalignant cohort. (B) Aplastic anemia (n = 1337). (C) Immune deficiency (n = 796). (D) Hemoglobinopathies (n = 656). (E) Histiocytic disorders (n = 432). (F) Bone marrow failure (n = 470). (G) Immunosuppressive conditioning (n = 759). (H) Reduced-intensity conditioning (n = 1820). (I) Myeloablative conditioning (n = 1439).
Univariable analysis showed that increasing HCT-CI scores were associated with decreased survival with hazard ratios (HRs) for associations with worse OS of 1.17 (95% CI, 0.98-1.39; P = .075) for scores 1 to 2; HR, 1.54 (95% CI, 1.28-1.86; P < .0001) for scores 3 to 4; and HR, 3.23 (95% CI, 2.58-4.06; P < .0001) for scores ≥5 compared with score 0. After adjusting for confounding variables by multivariable analysis (Table 2), HCT-CI scores ≥3 were associated with an increased hazard of death post-HCT compared with those without comorbidities. HCT-CI scores of 3 to 4 had a multivariable HR of 1.33 (95% CI, 1.09-1.63; P = .004), whereas scores ≥5 had a multivariable HR of 2.31 (95% CI, 1.79-2.96; P < .0001) compared with score 0. HR for HCT-CI scores of 1 to 2 were not significantly different than that of scores of 0 (HR, 1.12; 95% CI, 0.93-1.34; P = .218).
Multivariable Cox regression assessing HCT-CI and OS
| . | N . | HR (95% CI) . | P . |
|---|---|---|---|
| Entire nonmalignant cohort | |||
| 0 | 2512 | 1.00 | |
| 1-2 | 821 | 1.12 (0.93-1.34) | .218 |
| 3-4 | 556 | 1.33 (1.09-1.63) | .004 |
| ≥5 | 194 | 2.31 (1.79-2.96) | <.0001 |
| Aplastic anemia cohort | |||
| 0 | 726 | 1.00 | |
| 1-2 | 312 | 1.19 (0.87-1.64) | .275 |
| ≥3 | 299 | 2.06 (1.53-2.78) | <.0001 |
| Hemoglobinopathies cohort | |||
| 0 | 372 | 1.00 | |
| 1-2 | 157 | 0.46 (0.25-0.86) | .015 |
| ≥3 | 127 | 0.59 (0.29-1.21) | .153 |
| Immune deficiency cohort | |||
| 0 | 497 | 1.00 | |
| 1-2 | 174 | 1.37 (0.93-2.02) | .115 |
| ≥3 | 125 | 1.87 (1.21-2.88) | .0049 |
| Histiocytic disease cohort | |||
| 0 | 278 | 1.00 | |
| 1-2 | 77 | 0.89 (0.53-1.48) | .653 |
| ≥3 | 77 | 1.23 (0.78-1.92) | .374 |
| Bone marrow failure cohort | |||
| 0 | 320 | 1.00 | |
| 1-2 | 67 | 1.28 (0.76-2.17) | .358 |
| ≥3 | 83 | 1.80 (1.14-2.86) | .012 |
| Immunosuppressive conditioning | |||
| 0 | 451 | 1.00 | |
| 1-2 | 159 | 1.28 (0.78-2.11) | .315 |
| ≥3 | 143 | 1.58 (0.97-2.58) | .067 |
| Reduced-intensity conditioning | |||
| 0 | 1012 | 1.00 | |
| 1-2 | 408 | 1.00 (0.78-1.29) | .999 |
| ≥3 | 388 | 1.57 (1.24-2.00) | .0002 |
| Myeloablative conditioning | |||
| 0 | 1007 | 1.00 | |
| 1-2 | 239 | 1.26 (0.91-1.75) | .162 |
| ≥3 | 188 | 1.62 (1.18-2.22) | .003 |
| . | N . | HR (95% CI) . | P . |
|---|---|---|---|
| Entire nonmalignant cohort | |||
| 0 | 2512 | 1.00 | |
| 1-2 | 821 | 1.12 (0.93-1.34) | .218 |
| 3-4 | 556 | 1.33 (1.09-1.63) | .004 |
| ≥5 | 194 | 2.31 (1.79-2.96) | <.0001 |
| Aplastic anemia cohort | |||
| 0 | 726 | 1.00 | |
| 1-2 | 312 | 1.19 (0.87-1.64) | .275 |
| ≥3 | 299 | 2.06 (1.53-2.78) | <.0001 |
| Hemoglobinopathies cohort | |||
| 0 | 372 | 1.00 | |
| 1-2 | 157 | 0.46 (0.25-0.86) | .015 |
| ≥3 | 127 | 0.59 (0.29-1.21) | .153 |
| Immune deficiency cohort | |||
| 0 | 497 | 1.00 | |
| 1-2 | 174 | 1.37 (0.93-2.02) | .115 |
| ≥3 | 125 | 1.87 (1.21-2.88) | .0049 |
| Histiocytic disease cohort | |||
| 0 | 278 | 1.00 | |
| 1-2 | 77 | 0.89 (0.53-1.48) | .653 |
| ≥3 | 77 | 1.23 (0.78-1.92) | .374 |
| Bone marrow failure cohort | |||
| 0 | 320 | 1.00 | |
| 1-2 | 67 | 1.28 (0.76-2.17) | .358 |
| ≥3 | 83 | 1.80 (1.14-2.86) | .012 |
| Immunosuppressive conditioning | |||
| 0 | 451 | 1.00 | |
| 1-2 | 159 | 1.28 (0.78-2.11) | .315 |
| ≥3 | 143 | 1.58 (0.97-2.58) | .067 |
| Reduced-intensity conditioning | |||
| 0 | 1012 | 1.00 | |
| 1-2 | 408 | 1.00 (0.78-1.29) | .999 |
| ≥3 | 388 | 1.57 (1.24-2.00) | .0002 |
| Myeloablative conditioning | |||
| 0 | 1007 | 1.00 | |
| 1-2 | 239 | 1.26 (0.91-1.75) | .162 |
| ≥3 | 188 | 1.62 (1.18-2.22) | .003 |
For multivariable analysis on the entire cohort, the following variables were adjusted for in the final model: age, disease category, donor and graft source, recipient CMV status, and performance status. For multivariable analysis on subgroups by disease and conditioning intensity, the following variables were adjusted for in the final model: age, donor and graft source, recipient CMV status, and performance status.
Evaluation of HCT-CI by type of nonmalignant disease
In patients with acquired aplastic anemia, HCT-CI scores ≥3 were associated with decreased OS with an adjusted HR of 2.06 (95% CI, 1.53-2.78; P < .0001) and a lower 2-year OS of 67.6% (95% CI, 62.4-73.3) compared with those with no comorbidities who had a 2-year OS of 87.7% (95% CI, 85.1-89.9). The most common comorbidities in patients with aplastic anemia were pulmonary diseases (26%), hepatic diseases (11%), psychiatric diseases (10%), and infection (8%).
In patients with primary immune deficiencies, HCT-CI scores ≥3 had an adjusted HR of 1.87 (95% CI, 1.21-2.88; P = .0049) and 2-year OS of 65.5% (95% CI, 57.5-74.8), whereas patients without comorbidities had a 2-year OS of 84.3% (95% CI, 81.1-87.6; P < .0001). SCID represented the majority of diagnoses in this cohort (45%), followed by chronic granulomatous disease (17%), Wiskott-Aldrich syndrome (12%), and other primary immune deficiencies (26%). The most common comorbidity in patients with primary immune deficiencies was an ongoing infection at time of HCT, seen in 20% of patients. Pulmonary and hepatic diseases were also frequent in this group (12% and 11%, respectively).
In patients with congenital bone marrow failure syndromes, HCT-CI scores ≥3 were associated with decreased survival with an adjusted HR of 1.80 (95% CI, 1.14-2.86; P = .012) and 2-year OS of 61.4% (95% CI, 51.5-73.1) compared with patients with scores of 0 (2-year OS, 81.9%; 95% CI, 77.8-86.4; P < .0001). The most common comorbidities in this group were hepatic (14%) and pulmonary (13%) diseases.
In patients who received HCT for hemoglobinopathies, increasing HCT-CI scores were not associated with worse OS. The cohort includes 453 patients with sickle cell anemia, 165 with thalassemia, 32 with sickle thalassemia, and 6 with other hemoglobinopathies (supplemental Table 1). Patients with HCT-CI scores ≥3 had similar survival to those without comorbidities (HR, 0.59; 95% CI, 0.29-1.21; P = .153) on multivariable analysis. Two-year OS was similar among all groups. Patients with HCT-CI scores of 0 had an OS of 86.5% (95% CI, 82.9-90.2), similar to scores of 1 to 2 (92.8%; 95% CI, 88.9-97.0) and scores ≥3 (91.1%; 95% CI, 85.9-96.6). The most common comorbidities in this group were hepatic (23%), cerebrovascular (15%), and pulmonary (12%) diseases.
For patients who received HCT for histiocytic diseases, HCT-CI scores did not discriminate survival outcomes by multivariable analysis. but patients with scores ≥3 had a decreased 2-year OS of 60.4% (95% CI, 50.3-72.6) compared with patient scores of 0, who had a 2-year OS of 73.4% (95% CI, 68.3-78.8; P = .0136). In the group of patients with histiocytic disorders, the most common comorbidities included hepatic disease (18%) and infection (9%).
Evaluation of HCT-CI by age group
The cohort included 3195 pediatric patients <20 years of age and representing all diseases. Sixty-nine percent of pediatric patients had HCT-CI scores of 0, 19% had scores of 1 to 2, and 13% had scores ≥3. In pediatric patients, HCT-CI scores ≥3 were associated with an increased risk of mortality (HR, 1.61; 95% CI, 1.3-2; P < .0001). The remaining 888 patients were >20 years of age, and 36% had HCT-CI scores of 0, 26% had scores of 1 to 2, and 38% had scores ≥3. HCT-CI scores ≥3 were associated with increased mortality (HR, 2.01; 95% CI, 1.48-2.75; P < .0001). In both age groups, scores of 1 to 2 had similar survival outcomes to scores of 0 (1.08; 95% CI, 0.87-1.35) in pediatrics and HR, 1.42 (95% CI, 0.99-2.03) in adults.
Evaluation of HCT-CI by conditioning intensity
The predictive effect of HCT-CI on survival was observed in patients receiving both myeloablative and reduced-intensity conditioning regimens (Table 2; Figure 3). Patients receiving reduced-intensity conditioning with scores ≥3 (22% of reduced-intensity cohort) had an adjusted HR of 1.57 (95% CI, 1.24-2.00; P = .0002); patients receiving myeloablative conditioning with scores ≥3 (13% of myeloablative cohort) had an adjusted HR of 1.62 (95% CI, 1.18-2.22; P = .003) compared with score 0. Patients receiving reduced-intensity conditioning with scores ≥3 had a 2-year OS of 66.2% (95% CI, 61.4-71.2) compared with those with scores of 0 who had a 2-year OS of 80.7% (95% CI, 78.3-83.2), P < .0001. Patients receiving myeloablative conditioning with scores ≥3 had a 2-year OS of 72.7% (95% CI, 66.5-79.5) compared with those with scores of 0 with a 2-year OS of 81.3% (95% CI, 78.9-83.8), P = .0015.
In patients who received immunosuppressive regimens, survival estimates showed that 2-year OS survival could be stratified by HCT-CI score, with 91.1% (95% CI, 88.5-93.8) in those with scores of 0, 83.7% (95% CI, 78.0-89.8) in those with scores of 1 to 2, and 74.8% (95% CI, 67.7-82.6) in those with scores ≥3 (log-rank P < .0001). This included patients with aplastic anemia, congenital bone marrow failure syndromes, and primary immune deficiencies (supplemental Table 4). However, on multivariable analysis, HRs were similar in all HCT-CI groups (HCT-CI 0: HR, 1.00; HCT-CI 1 to 2: HR, 1.28 [95% CI, 0.78-2.11], P = .315; HCT-CI ≥3: HR, 1.58 [95% CI, 0.97-2.58], P = .067).
Causes of death after allogeneic HCT
There were 875 deaths after HCT (21% of patients). The most common reported causes of death were organ toxicity (34%) and infection (25%), regardless of HCT-CI scores (supplemental Table 5). Patients with HCT-CI scores ≥3 more frequently had disease recurrence reported as cause of death than patients with scores of 0 to 2 (22% vs 12%, respectively). Within each disease category, organ dysfunction followed by infection remained the most common cause of death (supplemental Table 6). GVHD was a common cause of death in patients with hemoglobinopathies (22% of deaths). Graft rejection, disease recurrence, or progression accounted for 30% of deaths in patients with metabolic diseases.
Discussion
This is the largest study to date evaluating the role of pretransplant comorbidity burden in predicting survival after HCT in patients with nonmalignant diseases. We report that the HCT-CI is valid in discriminating risks of mortality after HCT in this population, but appears to be disease-specific. The association between HCT-CI and mortality is mainly evident in acquired aplastic anemia, primary immune deficiencies, and congenital bone marrow failure syndromes, but not hemoglobinopathies. In addition, our cohort included a larger number of pediatric patients for HCT-CI assessment than has been previously described.15,16,25 We were able to show that patients with scores 1 to 2 tolerated HCT similar to those with score of 0 regardless of conditioning intensity. Finally, scores 3 to 4 and ≥5 represent 2 distinctive risk groups with increasing probabilities of mortality. Our results also reinforce post-HCT organ toxicity as an ongoing problem for patients with nonmalignant diseases, accounting for 34% of the deaths in our cohort. Overall, our findings have important implications for physicians in pre-HCT counseling, patient selection, and transplant approaches to minimize HCT-related risks for patients with nonmalignant diseases.
The concept of comorbidity presented here has been extrapolated from the management of malignant diseases, in which the presence of certain conditions may affect a patient’s ability to tolerate HCT and increase the risk of mortality. Patients with nonmalignant diseases present to HCT with a different comorbidity profile than patients with malignancies. Although pulmonary comorbidities remain the most common preceding condition for both nonmalignant (16%) and malignant diseases (23%),16 patients with nonmalignant diseases showed higher prevalence for hepatic disease (13%) and infection (8%). This may be due to disease-related end-organ damage (ie, hemoglobinopathies) or having a predisposition to developing infections (ie, primary immune deficiencies). These results suggest that comorbidities could be driven either by the primary disease itself or by disease-related therapies. Although some patients with nonmalignant diseases require HCT for survival (familial histiocytic diseases, SCID), the timing of HCT as the only potentially curative therapy for other severe nonmalignant diseases (hemoglobinopathies, aplastic anemia, metabolic diseases) can be variable. Our findings highlight the importance of taking into account baseline comorbidity burden when estimating the risk/benefit ratio and timing for HCT.
For patients with hemoglobinopathies, HCT-CI scores did not discriminate outcomes well, with similar survival rates irrespective of HCT-CI score. Although, the cohort predominantly comprises patients with sickle cell anemia, this finding was seen in both patients with sickle cell anemia and patients with thalassemia (supplemental Table 7). Several reasons and observations could be associated with this finding. Because patients with hemoglobinopathies had relatively fewer mortality events, an explanation is that, for this type of validation study, we do not have an adequately large sample size to demonstrate differences. Furthermore, patients with sickle cell disease who are referred to transplant often have declared themselves to have a severe phenotype, but such factors are not currently captured in the current form of the HCT-CI. For example, although pain crises are frequent, this comorbidity is not captured by the HCT-CI despite it being a sign of vascular disease. Thus, disease-related comorbidities associated with hemoglobinopathies, no matter how minor or severe, might actually benefit from HCT and resolve with cure of the underlying disease. We recognize that the hemoglobinopathy cohort in our study is unique in that the HCT-CI, in its current form, does not appear to predict risk. Additional future studies focused on this nonmalignant patient group, perhaps using additional data sets from European and Middle Eastern groups, will gain additional patient numbers and disease diversity to further determine if this lack of association is true or just by chance.
We acknowledge the limitations of this study; our data are supplied by different participating centers, where variability in assessments could be a problem. However, we believe this problem has been addressed by recent guidelines to help decrease the variability and better define HCT-CI comorbidities for patients.37 We also recognize the heterogeneity of this nonmalignant cohort with many underlying disease indications. To address this, we categorized the patients based on diagnosis and performed subgroup analyses. Given that certain diseases present to medical attention at different ages and that there are different rates of acceptance for the role of curative HCT, there is inherent confounding of age and disease.5,9,29 We decided to focus our subgroup analysis primarily on assessment of HCT-CI by disease indications because we believed that the comorbidities and HCT exposures would be similar within disease groups and adjusted for age in our regression model. We were able to validate the HCT-CI in patients with aplastic anemia, who generally present at older ages, as well as in patients with primary immune deficiencies, who present early in childhood. In addition, these disease categories include rare diseases that can only be evaluated in the setting of large registry studies. The distribution of diseases present in this cohort is representative of the population of patients with nonmalignant diseases treated with HCT worldwide and lends to the generalizability of our findings.38 Last, treatment approaches for patients with primary immune deficiencies, namely SCID, have likely changed in recent years since newborn screening was introduced in 2009. It is possible that, in our cohort, consequences of infection or infectious treatment contributed to the comorbidities present in this group and affected outcomes. Given the potential for earlier diagnosis from more widespread use of newborn screening, pre-HCT risk factors will need to be reevaluated in this unique population again in the future.
In conclusion, we have shown the HCT-CI to be an important tool for predicting risk of death after HCT in patients with nonmalignant diseases, except for patients with hemoglobinopathies. As the indications for undergoing HCT for patients with nonmalignant diseases expands, the utility of this tool in patient counseling will be critical, and future efforts should focus on exploring ways to decrease comorbidities or intervene with HCT before their onset. Future studies can aim to further refine pre-HCT risk assessment by combining HCT-CI with performance status, biomarkers, or other pre-HCT factors to better discriminate risk in patients with nonmalignant diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported primarily by a Public Health Service Grant/Cooperative Agreement from the National Institutes of Health, National Cancer Institute (NCI) (5U24CA076518), National Heart, Lung and Blood Institute (NHLBI), and National Institute of Allergy and Infectious Diseases; NHLBI and NCI grant/cooperative agreement (4U10HL069294); Health Resources and Services Administration (HHSH250201200016C); the Office of Naval Research (grants N00014-17-1-2388 and N0014-17-1-2850); and grants from Actinium Pharmaceuticals Inc, Amgen Inc,* Amneal Biosciences,* Angiocrine Bioscience Inc,* anonymous donation to the Medical College of Wisconsin, Astellas Pharma US, Atara Biotherapeutics Inc, Be the Match Foundation, bluebird bio Inc,* Bristol Myers Squibb Oncology,* Celgene Corporation,* Cerus Corporation, Chimerix Inc,* Fred Hutchinson Cancer Research Center, Gamida Cell Ltd, Gilead Sciences Inc, HistoGenetics Inc, Immucor, Incyte Corporation,* Janssen Scientific Affairs LLC, Jazz Pharmaceuticals Inc,* Juno Therapeutics, Karyopharm Therapeutics Inc, Kite Pharma Inc, Medac GmbH, MedImmune, The Medical College of Wisconsin, Mediware,* Merck & Co Inc,* Mesoblast,* MesoScale Diagnostics Inc, Millennium, Takeda Oncology Co, Miltenyi Biotec Inc,* National Marrow Donor Program, Neovii Biotech NA Inc,* Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Co Ltd.–Japan, PCORI, Pfizer Inc,* Pharmacyclics LLC,* PIRCHE AG, Sanofi Genzyme,* Seattle Genetics,* Shire, Spectrum Pharmaceuticals Inc, St Baldrick’s Foundation, Sunesis Pharmaceuticals Inc,* Swedish Orphan Biovitrum Inc, Telomere Diagnostics Inc, and University of Minnesota. This publication was supported by grants from the National Institutes of Health, National Center for Advancing Translational Sciences (UL1TR001436 and 1TL1TR001437) (L.B.) and the Midwest Athletes Against Childhood Cancer Fund (M.S.T.). *Corporate members.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US Government. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: M.S.T., M.C.P., and M.L.S. designed the study; L.B., C.F., and B.L. performed data analysis; L.B. and M.S.T. wrote the initial draft of the manuscript which was edited by all authors; and all authors agreed to the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mohamed L. Sorror, Transplantation Biology, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, Seattle, WA 98109; e-mail: msorror@fredhutch.org.
REFERENCES
Author notes
M.S.T. and L.B. are joint first authors.
M.C.P. and M.L.S. are joint last authors.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal