TO THE EDITOR:
Osteonecrosis is a frequent complication of therapy in patients with acute lymphoblastic leukemia (ALL), particularly children aged 10 years and older at diagnosis and young adults.1,,,,,-7 Prior clinical8 and nonclinical9 data have demonstrated that arteriopathy in arterioles supplying the bone and marrow, characterized by vascular smooth muscle and endothelial cell injury, is the initiating lesion in the development of glucocorticoid-induced osteonecrosis. In patients with ALL, osteonecrosis has been linked to receptors for the vascular toxin glutamate.1 We therefore hypothesized that patients may exhibit other evidence of endothelial dysfunction and vascular toxicity before the onset of osteonecrosis. Because hypertension is a known adverse effect of glucocorticoid therapy10,11 and has been linked to adverse vascular remodeling,12,13 we hypothesized that hypertension represents an early manifestation of therapy-induced vascular dysfunction, which ultimately contributes to osteonecrosis.
We evaluated this in 254 children aged from 10 to 18.99 years who were enrolled on the Total Therapy XV14 (NCT00137111) and XVI15 (NCT00549848) studies, identifying hypertension during either induction or reinduction, as defined by the American Academy of Pediatrics fourth report on pediatric hypertension.16 Patients with ALL were diagnosed with osteonecrosis by magnetic resonance imaging (supplemental Methods, available on the Blood Web site).17 Only patients with osteonecrosis symptoms (Common Terminology Criteria for Adverse Events [CTCAE] grade 2 or higher) were considered cases for the primary analysis. All studies were approved by the institutional review board. Age-appropriate informed consent/assent was obtained as specified by the Declaration of Helsinki.
Among the 235 evaluable children (supplemental Figure 1; supplemental Table 1), 94 were categorized as hypertensive. Treatment on the Total XVI protocol was associated with a decreased incidence of hypertension (30.6% vs 54.9% in Total XV; P = 2.4 × 10−4), and patients of mixed or Asian ancestry had lower rates of hypertension than those of white ancestry (14.3% vs 43.5%; P = .0499); no other clinical factors were associated with the development of hypertension, including sex, age, body mass index z-score, or risk group.
Symptomatic osteonecrosis was diagnosed in 103 patients. Osteonecrosis was more common in girls than in boys (P = .014), and more common in ancestral whites than in ancestral blacks (P = .016). There was no association between osteonecrosis and age (P = .36), risk group (P = .16), treatment protocol (P = .98), or body mass index z-score (P = .06).
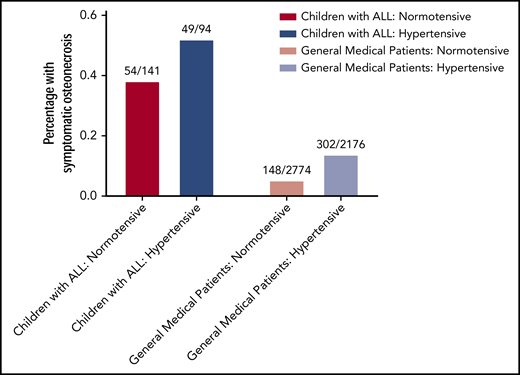
Hypertension was associated with an increased risk for symptomatic osteonecrosis (49/94 vs 54/141 in normotensive patients [P = .037]; odds ratio [OR], 1.75; 95% confidence interval [CI], 1.02-3.01). In a multivariable analysis including sex, protocol, and ancestry, hypertension’s association with increased osteonecrosis risk strengthened (P = .03; OR, 1.88; 95% CI, 1.1-3.22; Figure 1; supplemental Table 2). Hypertension was also associated with more radiographically extensive epiphyseal osteonecrosis after reinduction 1 therapy (P = .021; supplemental Methods; supplemental Table 3), although it was not associated with osteonecrosis when cases included asymptomatic osteonecrosis (CTCAE grade 1 or higher) measured at any point during the protocol (P = .38; supplemental Methods; supplemental Table 4).
Hypertension is associated with an increased risk for osteonecrosis. In children aged 10 years and older diagnosed with ALL, those with hypertension (dark blue bar) had an increased risk of developing osteonecrosis later in therapy compared with normotensive (red bar) patients (52.1% vs 38.3%; multivariate P = .03; odds ratio, 1.88, left). In a general medical population receiving glucocorticoids for any diagnosis, hypertension during glucocorticoids was also associated with an increased risk of developing osteonecrosis (13.9% vs 5.3%; multivariate P = 7.46 × 10−21; odds ratio, 2.85, right).
Hypertension is associated with an increased risk for osteonecrosis. In children aged 10 years and older diagnosed with ALL, those with hypertension (dark blue bar) had an increased risk of developing osteonecrosis later in therapy compared with normotensive (red bar) patients (52.1% vs 38.3%; multivariate P = .03; odds ratio, 1.88, left). In a general medical population receiving glucocorticoids for any diagnosis, hypertension during glucocorticoids was also associated with an increased risk of developing osteonecrosis (13.9% vs 5.3%; multivariate P = 7.46 × 10−21; odds ratio, 2.85, right).
To validate the association of symptomatic osteonecrosis with hypertension, we evaluated records of 4950 general medical patients from the Vanderbilt Synthetic Derivative,18 using a previously validated strategy.1 All patients received at least 2 weeks of systemic glucocorticoids. Four hundred fifty cases of osteonecrosis were identified by International Classification of Diseases, Ninth Revision/10th Revision (ICD9/10), codes and manually confirmed. Control individuals (without ICD9/10 codes or keywords for osteonecrosis) were selected in a 10:1 ratio with case patients and matched on sex, race, and ethnicity. Hypertension (defined by ICD9/10 codes) was categorized according to timing relative to glucocorticoid administration as before, during, both, or neither.
Of the 4950 steroid exposed individuals, 2176 were diagnosed with hypertension on glucocorticoids. Medical patients who developed osteonecrosis were older (median age, 57 years) than those who did not develop osteonecrosis (median age, 53 years; P = 2.5 × 10−4).
Similar to the ALL cohort, patients diagnosed with hypertension during glucocorticoids had an increased risk for osteonecrosis (302/2176 vs 148/2774 in normotensive patients; P = 1.18 × 10−23; OR, 2.86; 95% CI, 2.32-3.53; supplemental Table 5). Patients diagnosed with hypertension before glucocorticoids experienced a smaller increased risk (P = .015; OR, 1.32; 95% CI, 1.05-1.65). The association between hypertension and osteonecrosis was maintained in multivariable analysis including age (P = 7.46 × 10−21; Figure 1), and in the 3865 patients without preglucocorticoid hypertension (P = 9.54 × 10−22; OR, 3.06; 95% CI, 2.43-3.87).
To test whether antihypertensive therapy could prevent the increased risk for osteonecrosis, we tested the effect of antihypertensives, using the murine model of osteonecrosis.9 All mice received dexamethasone 2 mg/L in drinking water continuously, and PEGaspargase 1200 IU/kg intraperitoneal weekly, beginning at 26 to 28 days of life and continuing for 6 weeks. Mice were also randomly assigned to receive quinapril hydrochloride 60 mg/L in drinking water continuously vs control or, in a second experiment, hydralazine 320 mg/L vs control. At the completion of therapy, mice were humanely euthanized and were evaluated for osteonecrosis and epiphyseal arteriopathy in the hind limbs, as described.9 Blood pressures were measured via tail cuff in identically treated mice. All animal experiments were approved by the Institutional Animal Care and Use Committee (supplemental Methods).
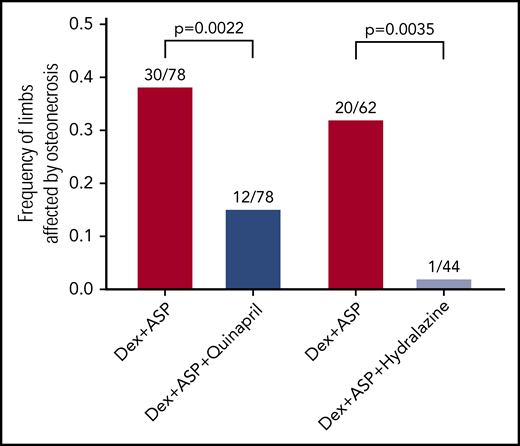
Compared with control mice treated with chemotherapy only, mice treated with chemotherapy and quinapril were less likely to have osteonecrosis (10/39 vs 26/39 mice; P = 6.6 × 10−4) and had fewer hind limbs affected by osteonecrosis (12/78 vs 30/78; P = .002; Figure 2). Quinapril did not reduce the development of epiphyseal arteriopathy in evaluable limbs (23/74 vs 32/75; P = .2), but significantly reduced the progression from arteriopathy to osteonecrosis (12/23 vs 28/32; P = .009). Hydralazine similarly decreased the frequency of osteonecrosis (1/22 vs 17/31 mice; P = 4.4 × 10−4) and decreased the number of affected limbs (1/44 vs 20/62; P = .0035; Figure 2). Hydralazine also decreased the frequency of arteriopathy (2/40 vs 30/59 in chemotherapy control; P = 3.4 × 10−6).
Antihypertensives decrease osteonecrosis resulting from chemotherapy. Mice treated with dexamethasone and asparaginase (Dex+ASP, red) for 6 weeks develop osteonecrosis. Mice who received quinapril in addition to dexamethasone and asparaginase (Dex+ASP+Quinapril, dark blue) developed less osteonecrosis (12/78 vs 30/78; P = .0022). In a second experiment, hydralazine given with dexamethasone and asparaginase (Dex+ASP+Hydralazine, light blue) also decreased osteonecrosis compared with chemotherapy alone (1/44 vs 20/62; P = .0035).
Antihypertensives decrease osteonecrosis resulting from chemotherapy. Mice treated with dexamethasone and asparaginase (Dex+ASP, red) for 6 weeks develop osteonecrosis. Mice who received quinapril in addition to dexamethasone and asparaginase (Dex+ASP+Quinapril, dark blue) developed less osteonecrosis (12/78 vs 30/78; P = .0022). In a second experiment, hydralazine given with dexamethasone and asparaginase (Dex+ASP+Hydralazine, light blue) also decreased osteonecrosis compared with chemotherapy alone (1/44 vs 20/62; P = .0035).
As in patients, dexamethasone/asparaginase chemotherapy induced hypertension. Median systolic pressure was 139.4 mm Hg in treated mice (N = 6) compared with 117 mm Hg in untreated mice (N = 6; P = 1.4 × 10−5). Mice receiving dexamethasone and asparaginase had attenuated systolic hypertension when treated with either quinapril (N = 6; median, 127.2 mm Hg; P = .011) or hydralazine (N = 8; median, 123.2 mm Hg; P = .012; supplemental Figure 2).
There was no effect of antihypertensives on the antileukemic efficacy of therapy in vitro or in vivo (supplemental Materials and Results).
We reviewed records of patients with ALL and noted that antihypertensive use (N = 50 patients) was not associated with an altered risk for osteonecrosis (P = .32). The retrospective nature of the data makes the reason for this discrepancy unclear, although it appears patients treated for hypertension were more hypertensive, treated briefly, and had only moderate changes in blood pressure during antihypertensive therapy (see supplemental Data for further details and discussion). Osteonecrosis also developed in many patients without documented hypertension, suggesting additional factors contribute to osteonecrosis development, although incomplete ascertainment of hypertension may have also occurred in these retrospective cohorts. Although hypertension was not associated with osteonecrosis when asymptomatic cases were included, it was associated with both symptomatic osteonecrosis (CTCAE grade 2 or higher) and extensive epiphyseal osteonecrosis. Although the reason for this discrepancy is unclear, our murine data indicate that antihypertensive therapy may affect epiphyseal osteonecrosis, the lesions most correlated with joint collapse.19
Our data demonstrate that patients who develop hypertension during glucocorticoid containing portions of ALL therapy are at increased risk of developing symptomatic osteonecrosis, although the retrospective nature of this study makes causal determination difficult. Nonclinical data demonstrate that antihypertensives can reduce the risk for osteonecrosis, but that they must effectively decrease hypertension to affect osteonecrosis. These findings align with data demonstrating a benefit to deeper antihypertensive control in diverse adult20,21 and pediatric22 populations. Further studies are needed to determine whether similar control of hypertension will decrease osteonecrosis in patients receiving ALL therapy.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Monique Payton, David Jenkins, Jean Cai, Nagasai Adusumilli, and Keith Young for assistance with experiments; Wenjian Yang, Colton Smith, and Nancy Kornegay for bioinformatics support; Janey Wang for support with the Vanderbilt Synthetic Derivative cohort; and the Veterinary Pathology Core and Animal Resource Center of St. Jude for assistance with animal experiments. The authors also thank the patients and their families for their participation.
Funding provided by a Hyundai Hope on Wheels Young Investigator Grant, American Lebanese Syrian Associated Charities, Burroughs Wellcome Fund IRSA 1015006, the National Institutes of Health, National Cancer Institute (CA142665, CA21765, CA23944) and the National Institutes of Health, National Institute of General Medical Sciences (GM115279). Data were included from Vanderbilt University Medical Center’s Synthetic Derivative, which is supported by institutional funding and by Clinical and Translational Science Awards grant ULTR000445 from the National Institutes of Health, National Center for Advancing Translational Sciences.
Authorship
Contribution: L.J.J., S.L.V.D., J.C.D., M.V.R., and S.E.K. designed the studies; S.J. and C.-H.P. designed and administered the clinical trials; L.J.J., S.L.V.D., M.V.P., R.V.A., S.C.K., and S.E.K. performed data collection; D.P., C.C., and S.E.K. performed data analysis; S.E.K. created figures; S.L.V.D., D.P., C.C., and S.E.K. had access to raw data; and all authors contributed to manuscript writing and review.
Conflict-of-interest disclosure: S.L.V.D. reports grants from Burroughs Wellcome Fund during the conduct of the study, and honorarium from Merck outside the submitted work. R.V.A. reports ownership in Prediction Health Inc. outside the submitted work. M.V.R. reports grants from the National Institutes of Health during the conduct of the study and grants from Servier outside the submitted work. H.I. reports grants from Servier outside the submitted work. S.E.K. reports grants from Hyundai Hope on Wheels during the conduct of the study. The remaining authors declare no competing financial interests.
Correspondence: Seth E. Karol, Leukemia & Lymphoma Division, Department of Oncology, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS 260, Memphis, TN 38105; e-mail: seth.karol@stjude.org.