Background: Red blood cell (RBC) alloimmunization can follow exposure to foreign RBC antigens during transfusion therapy and during pregnancy/delivery. Alloantibodies against RBC antigens can cause potentially fatal hemolytic transfusion reactions and hemolytic disease of the newborn. Recent studies indicate that inflammatory states, including autoimmunity, promote RBC alloimmunization. For example, patients with systemic lupus erythematosus (SLE) have an elevated risk of RBC alloimmunization. Approximately 50% of SLE patients have a pro-inflammatory type 1 interferon (IFNα/β) gene signature, defined as the expression of interferon stimulated genes (ISGs), that is associated with disease severity. Recent studies in mouse transfusion models indicate that IFNα/β significantly promotes RBC alloimmunization. Thus, we hypothesized that IFNα/β produced in a lupus mouse model would promote RBC alloimmunization following transfusion.
Methods: To test this hypothesis, we utilized the pristane-induced lupus mouse model, known to manifest lupus autoantibodies and a lupus-like phenotype. Mice lacking the IFNα/β receptor (IFNAR1-/-), mice lacking transcription factors required for IFNα/β production (IRF3/7-/-) and wildtype (WT) mice were administered an intraperitoneal injection of pristane 14 days prior to transfusion with transgenic RBCs expressing the KEL antigen. At the time of transfusion, serum IFNα levels were measured by ELISA, and expression of interferon stimulated gene 15 (ISG15) was measured by quantitative PCR. The mean fluorescence intensity (MFI) of the anti-KEL IgM and IgG responses were measured by flow cytometry 5 days and 7-28 days, respectively, following transfusion. A Mann-Whitney U test and a Kruskall-Wallis test with a Dunn's post-test was used to compare 2 or more than 2 groups, respectively.
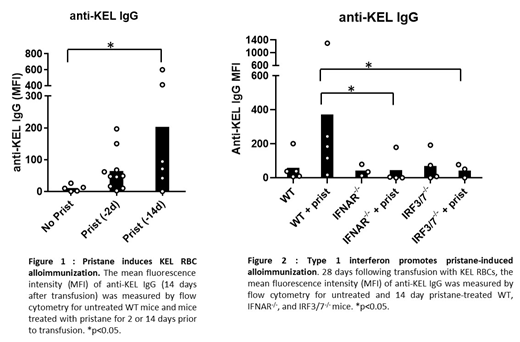
Results: Pristane induced serum IFNα in WT mice (Day 14 post-pristane, mean IFNα=127.3 Units/ml ± 28.21 standard error of the mean, SEM, p<0.01), which was undetectable in untreated mice. Pristane treatment also resulted in a non-significant trend toward upregulated expression of ISG15, post-pristane ISG15 =2.94 ± 1.16 SEM; no pristane ISG15 = 1.4 ± 0.2 SEM). Following transfusion, untreated mice did not form anti-KEL IgG. However, pristane-treated WT mice produced significantly elevated levels of anti-KEL IgM and anti-KEL IgG in 3 out of 3 experiments (untreated IgM MFI = 79.1 ± 13.5, pristane-treated IgM MFI = 142.7 ± 21.6, p<0.01; untreated IgG MFI = 10.7± 4.6, pristane-treated IgG MFI= 203.9 ± 99.6, p<0.05, Figure 1). Pristane-treated IFNAR1-/- and IRF3/7-/- mice produced significantly lower levels of anti-KEL IgG (IFNAR1-/- MFI = 39.0 ± 35.6, p<0.05; IRF3/7-/- MFI = 36.1 ± 16.3, p<0.02) compared to WT mice (MFI = 361 ± 148).
Conclusion: Pristane induction of a lupus-like phenotype promoted alloimmunization to the KEL RBC antigen in an IFNα/β-dependent manner. These results warrant further investigation of the role of IFNα/β in alloimmunization to other RBC antigens, and the investigation of the contribution of IFNα/β responses to increased frequency of alloimmunization in patients with SLE.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal