Heparin induced thrombocytopenia (HIT) is a severe adverse reaction to heparin treatment characterized by antibodies to PF4/polyanion complexes. However, HIT antibodies are heterogeneous. Some bind to heparin/polyanion complexes in solid-phase enzyme-linked immunoassay (EIA) but do not activate platelets; other EIA-positive antibodies also activate platelets in assays such as the serotonin release assay (SRA) and in the recently described PF4-dependent p-selectin expression assay (PEA). It is generally thought that only platelet-activating antibodies are pathogenic.
On the basis of studies which showed that platelet-activating antibodies, but not those positive only in EIA recognize PF4 bound to platelets (Blood. 2015;125(1):155-61 and others), we hypothesized that pathogenic HIT antibodies may recognize epitopes exposed on PF4 when it binds to a target, possibly chondroitin sulfate, on the platelet surface that are not created when PF4 forms a complex with heparin or other polyanions. Here, we describe studies utilizing newly developed monoclonal antibodies (mAbs) that demonstrate the feasibility of this concept and suggest that HIT antibodies having this specificity could be important in HIT pathogenesis.
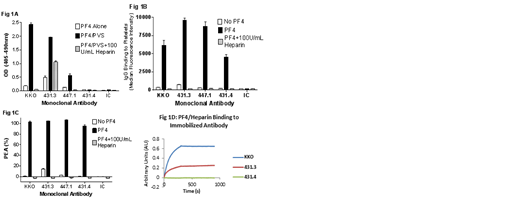
mAbs were produced in mice immunized with PF4/heparin complexes. We employed a novel dual screening strategy for candidate clones utilizing the PF4/polyanion EIA as well as the PEA. mAbs selected for study were designated 431.3, 431.4 and 447.1 and were compared with KKO, a widely studied HIT-like mAb (generous gift from G Arepally) and an isotype control (IC; Fig 1A). In the EIA, KKO strongly recognized PF4/polyvinylsulfonate (PF4/PVS) but not PF4 alone and was inhibited by high-dose heparin (100U/mL). mAb 447.1 had an EIA profile similar to KKO but with significantly lower optical densities (0.56 vs. 2.43 OD) and 431.3 gave reactions indicating it recognizes PF4 alone but demonstrated much stronger reactivity to PF4/PVS complexes (1.96 OD). However, mAb 431.4, similar to isotype control, was completely non-binding in the EIA (Fig 1A). In contrast, all 4 mAbs strongly recognized PF4-treated platelets (Fig 1B). In addition, all four mAbs produced strong positive reactions in the PEA in which mAbs interact with PF4-primed platelets to induce p-selectin expression (Fig 1C). The EIA requires multiple washing steps, whereas studies performed to assess IgG binding to PF4-treated platelets utilized only a single wash and the PEA is a "no-wash" assay. Thus, failure of 431.4 to react in EIA could be explained by susceptibility of the mAb to being removed by washing, possibly due to low avidity for its target. We therefore studied mAb binding to PF4/heparin complexes in real time using Bio-layer interferometry (Octet system, Pall ForteBio). In this system, mAbs 431.4 and KKO produced a steadily increasing signal, reaching saturation, whereas 431.4 demonstrated no binding to PF4/heparin consistent with results seen in EIA (Fig 1D).
These findings indicate that mAb 431.4 binds to PF4 bound to the platelet surface and induces platelet activation as measured by p-selectin expression but fails to react with PF4 in a complex with heparin or PVS. This behavior contrasts strikingly with that of the "HIT-like" mAb KKO that reacts in both assays. Although antibodies from patients with HIT frequently give positive reactions in both platelet-activating assays and in EIA, there are clear examples of antibodies that are positive in one assay and not the other (e.g. EIA-positive, SRA-negative antibodies are common in clinical practice). Thus in HIT patients, multiple antibodies with different specificities are undoubtedly present. Our data support the growing body of evidence that while strength of the reaction in EIA (optical density) is often correlated with platelet-activating antibodies, there are notable exceptions (such as mAb 447.1). These results suggest that studies to isolate and characterize human antibodies according to specificity and reaction strength are needed to define the extent to which antibodies like mAb 431.4 and 447.1 contribute to the pathogenesis of HIT.
Padmanabhan:Versiti Wisconsin: Patents & Royalties: Related to HIT patents; Retham Technologies: Equity Ownership; Terumo BCT: Consultancy; Veralox Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen R&D: Consultancy. Jones:Retham Technologies: Equity Ownership; Versiti Wisconsin: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal