Aplastic anemia (AA) is a hematopoietic disorder resulted from immune-related hypocellular hematopoiesis in bone marrow (BM). It has been clearly addressed that the activated T cells contribute to the exhaustion of hematopoietic progenitors and hypo-hematopoiesis. The adipogenic BM is one of the characteristics to make AA diagnosis. However, little is known about the relationship of intra-BM immune imbalance and hematopoietic microenvironment abnormity in this disease entity.
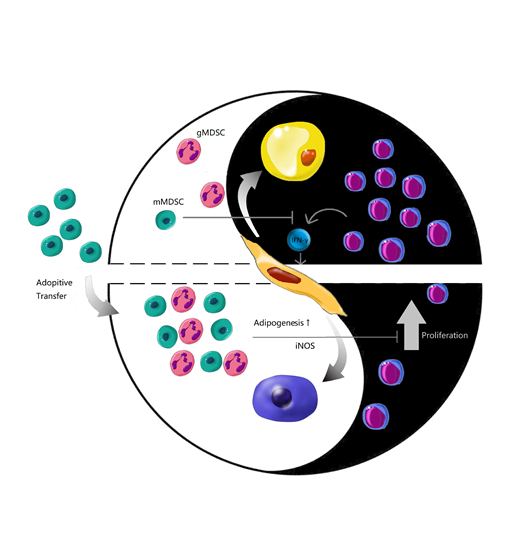
Functional hematopoiesis relies on not only abundant hematopoietic stem cells (HSCs) but also the balanced supportive hematopoietic niche. Intra-BM immune balance, at either cellular or cytokine level, is one of the key footstones to maintain hematopoietic microenvironment. Various intra-BM immune cellular components play both sides of one coin. Among them, myeloid-derived suppressive cells (MDSCs) are heterogeneous myeloid progenitor cells characterized by the negative immune response in cancers and other inflammatory diseases. In BM aspiration and biopsy samples from the patients who were diagnosed as AA in our study, massive activated lymphocytes infiltration and adipocytes accumulation were observed. Interestingly, the absolute numbers of immune modulatory MDSCs either in AA patients' PB or in BM of immune-related AA mice were reduced, indicating a potential link between polarized BM adipo-osteogenic microenvironment and immune disorder under AA circumstance. We thus adopted AA mice model to look into the embedded details both in vivo and in vitro.
We clarified that BM components were more vulnerable to the attack of CD8+ T cells than that of CD4+ T cells. Taking into the fact that BM adipocytes are more abundant either in AA patients or in AA mice models, we differentiated mesenchymal stromal cells (MSCs), the major BM stroma cells, into osteoblastic or adipogenic lineages to mimic the osteo-adipogenic differentiation in BM microenvironment. Interestingly, CD8+ T cells and interferon-γ(IFN-γ) exerted dramatically adipocytic stimulation on BM-MSCs either in vitro or in vivo, by determination of increasing expression of adipogenetic genes including Ap2, Perilipin, Pparg and Cebpα, as well as staining of Oil Red O and perilipin.
To dissect intra-BM cellular immune balance, MDSCs were isolated as representative immune regulating population to investigate their function on osteo-adipogenic balance. Interestingly, not CD11b+Ly6G+Ly6C-granulocytic-MDSCs (gMDSCs) but CD11b+Ly6G-Ly6C+monocytic-MDSCs (mMDSCs) inhibited both T cell proliferation and IFN-γ production. Addition of L-NMMA, the antagonist of iNOS pathway in mMDSCs-containing system restored T cell proliferative curve and cell numbers, whereas Nor-NOHA, the antagonist of Arg-1 pathway didn't abrogate mMDSCs' immune-regulation properties, indicating that mMDSCs inhibited T cell proliferation via iNOS pathway.
We then performed single dose or multi-dose injection of mMDSCs in AA mice to see whether mMDSCs are able to reconstitute the impacted hematopoiesis. Single injection of mMDSCs was able to prevent from CTL infiltration in a very short term. However, multi-injection of mMDSCs showed significant benefit in overall survival rate compared to AA mice. We further detected the function of mMDSCs on polarized BM-MSCs adipo-osteogenic differentiation potential. To detect sequential BM adipogenetic progression in AA microenvironment, we performed in vivo fluorescent microscopy on AP2 (Fabp4)-Cre×mT/mG reporting mice at different transfusion time points of T cells and mMDSCs. GFP-expressing AP2+ adipocytes accumulated adjacently to perivascular niches whose boarders were labelled by Dextran-CY5 in a time-dependent manner after T cell infusion. Monocytic MDSCs transfused AA mice showed decreased GFP+ adipocytes which was coincident with our in vitro findings.
In conclusion, intra-BM immune balance is one of the environmental factors seesawing by activating and suppressive ends to support functional hematopoiesis. Adoptive transfusion of mMDSCs, the immune-suppressive population might be a novel immune-regulating strategy to treat AA, relying on not only restoring the intra-BM immune balance but also improving stroma's multi-differentiating microenvironment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal