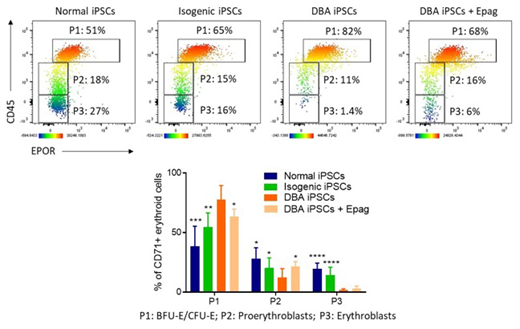
Diamond Blackfan Anemia (DBA) is a congenital bone marrow (BM) failure syndrome primarily characterized by defective erythropoiesis. In most patients, pathogenic heterozygous mutations have been identified in genes encoding ribosomal proteins (RP). The resulting RP haploinsufficiency was recently shown to delay globin protein translation in erythroid cells, whereas synthesis of heme, the nonprotein iron-containing component of hemoglobin, proceeds normally (Yang et al., Sci Transl Med 2016). Because heme is first synthesized at or just before the proerythroblast stage, free heme is in excess of globin in these cells. High levels of free heme induce proerythroblast cell death, and erythroid differentiation thus halts at the earlier BFU-E/CFU-E progenitor stage. Consistent with these observations, inhibition of heme synthesis with succinylacetone was previously shown to improve erythroid differentiation of DBA marrow cells in vitro. In this study, we investigated whether eltrombopag (Epag), an FDA-approved mimetic of thrombopoietin that promotes trilineage hematopoiesis in subjects with acquired BM failure (Olnes et al., NEJM 2012; Townsley et al., NEJM 2017), could rescue erythropoiesis in DBA. We hypothesized that Epag might inhibit heme synthesis by restricting iron availability due to its robust intracellular iron chelating properties, leading to decrements in iron-induced reactive oxygen species (ROS) and increased proerythroblast survival and maturation. To test this possibility,we first established an induced pluripotent stem cell (iPSC) model of DBA by reprogramming mononuclear cells (MNCs) from a patient with inactivating mutations in RPS19, the most commonly mutated gene in DBA. We also generated a control isogenic iPSC line by CRISPR/Cas9-mediated correction of RPS19 point mutations in the established DBA iPSC line.RPS19 haploinsufficiency was confirmed by Western blot and the expected reduction in 40S/60S ribosomal subunit ratio was detected by polysome profiling of DBA iPSCs. This phenotype normalized in the isogenic iPSCs. Both DBA and isogenic iPSC lines, and iPSCs derived from a healthy donor, were then subjected to hematopoietic differentiation for 21 days using the STEMdiffTMmonolayer-based approach (Ruiz et al., BioRxiv 2019). Hematopoietic cells were harvested between day 19 and 21 of culture when maximum erythroid production is observed in this system. Normal and isogenic iPSCs efficiently gave rise to erythroid cells at various stages of maturation, including CD71+CD45+EPOR+primitive erythroid progenitors (P1), CD71+CD45loEPOR-proerythroblasts (P2), and more mature CD71+CD45-EPOR-erythroblasts (P3) (Figure). In contrast, the majority of erythroid cells detected after differentiation of DBA iPSCs were comprised within P1 with limited maturation to P2 and P3, consistent with a block in differentiation at the early erythroid progenitor stage (Figure). Furthermore, in colony forming unit (CFU) assays, DBA iPSCs generated numbers of myeloid colonies (CFU-G, CFU-M and CFU-GM) comparable to normal and isogenic iPSCs, but erythroid colonies (BFU-E and CFU-E) were undetectable, in keeping with DBA progenitor's inability to differentiate in vitro. Next, DBA iPSCs were differentiated in the presence of Epag 3 µg/mL from day 10 to 21 of culture. Addition of Epag improved late erythroid maturation, as indicated by reduced percentages of early progenitors (P1) and a concomitant increase in more mature P2 and P3 erythroblastic populations (Figure). Investigations are ongoing to confirm Epag-mediated iron restriction and decreased heme synthesis as the primary molecular mechanism underpinning the improved erythroid maturation observed in this study. Overall, our data indicate that directed differentiation of DBA iPSCs recapitulates early erythroid maturation defects in vitro, and erythropoiesis can be rescued in part by addition of Epag during culture. These results suggest that Epag may improve red blood cell production in patients with DBA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal