Background
CD33 (Siglec3) is a cell surface transmembrane receptor that is rapidly internalized and highly expressed on AML blasts but is absent on normal hematopoietic stem cells. Gemtuzumab ozogamicin (GO), a humanized anti-CD33 antibody conjugated to a DNA strand scission inducing agent (calicheamicin) was recently FDA approved for the treatment of newly-diagnosed or relapsed/refractory CD33-positive acute myeloid leukemia (AML). GO has been shown to exert clinical activity in leukemia patients. Poly (ADP-ribose) polymerase (PARP) inhibitors prevent the repair of single stranded DNA breaks by blocking the nicotinamide adenine dinucleotide (NAD) catalytic domain of the PARP protein and by preventing the dissociation of PARP from the DNA (PARP trapping). Talazoparib is unique among clinical PARP inhibitors in displaying 10,000-fold increased PARP trapping as compared to other agents . We hypothesized that combination therapy using GO and Talazoparib would result in synergistic anti-leukemic effects on human CD33+ AML cells due to the ability of the PARP inhibitor to enhance levels of DNA damage induced by GO therapy.
Materials and Methods
Human AML cell lines were characterized for CD33 expression using flow cytometry after staining with antibody-linked fluorescent QuantiBrite Beads. Cells were continuously exposed to varying doses of GO (10pM - 100mM) and PARP inhibitors (1nM - 100mM) for 96h alone and in combination. Cell viability was measured immediately following treatment using a WST colorimetric assay. Treatment-induced apoptosis (annexin/PI) and DNA damage (H2AX) were quantified by flow cytometric assays. Synergy reports were generated using Compusyn software. In vivo efficacy was assessed in NSG mice systematically engrafted with luciferase labeled human CD33+ AML cells following tail vein injection. Animals were treated with varying doses of vehicle, GO (1 and 50ug/kg 1x/week for 3 weeks), or talazoparib (0.1 and 0.33mg/kg 5 days/week) either alone or in combination. Treatment effects on leukemia burden, toxicity, and survival were determined by weekly whole animal bioluminescent imaging, total animal weights, and time to morbidity.
Results
Human AML cell lines (HEL, HL60) express high expression levels of CD33 molecules/cell (43,645 and 31,286 respectively) relative to negative controls. Continuous exposure to single agent GO and Talazoparib for 96h resulted in a dose dependent inhibition of human AML cell growth (HEL, HL60) . IC values for GO were 0.01 - 6.6μg/ml and for Talazoparib were 0.8-0.95μM. Combination in vitro therapy with GO (0.005 - 1μg/ml) and Talazoparib (fixed dose 100nM) resulted in synergistic anti-leukemic effects (p<0.01) significantly improving upon monotherapy. Software analyses yielded a combination Index (CI) <1 consistent with synergistic anti-leukemic effects. Combination GO and Talazoparib therapy also significantly enhanced AML cell apoptosis (p=0.0111) and levels of DNA damage (phosphorylated H2AX) (p=0.0054) over single agent activity. Evaluation of PARP trapping by western blot analysis is ongoing.
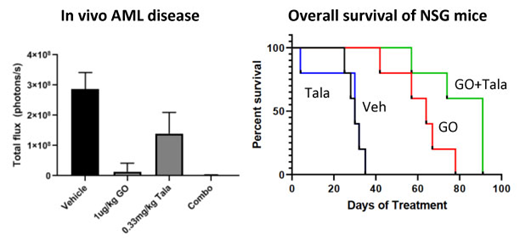
In vivo administration of GO (1-50μg/kg) and Talazoparib (0.1-0.33mg/kg) in NSG mice with systemic engraftment of luciferase tagged human CD33+ AML cells was generally well tolerated with no significant weight loss or early morbidity. Single agent GO and Talazoparib therapy decreased systemic AML burden in a dose dependent manner and prolonged overall survival over vehicle treated mice (P<0.05). Concomitant GO (1μg/kg) and Talazoparib (0.33μg/kg) treatment was similarly well tolerated with no notable weight loss or toxicities. Combination GO and Talazoparib therapy significantly prolonged overall survival of leukemia xenografted mice over vehicle (p=0.0018) and single agent therapy with the same doses of GO (p=0.0018) and Talazoparib (p=0.0499), respectively).
Conclusions
In summary, our results demonstrate that the addition of the PARP inhibitor, Talazoparib, to the CD33 antibody drug conjugate, GO, results in potent in vitro and in vivo anti-tumor activity in human CD33+ AML preclinical models. Further studies investigating this novel combinatorial approach in AML are currently ongoing. Due to GO's FDA approval for CD33+ AML in 2018, this data strongly supports future clinical investigation using PARP inhibitors as a novel class of agents for combination therapy to significantly enhance the efficacy of ADCs.
Wang:Amgen: Other: Advisory role; Agios: Other: Advisory role; Stemline: Other: Advisory role, Speakers Bureau; Daiichi: Other: Advisory role; Abbvie: Other: Advisory role; Kite: Other: Advisory role; Jazz: Other: Advisory role; Astellas: Other: Advisory role, Speakers Bureau; celyad: Other: Advisory role; Pfizer: Other: Advisory role, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal