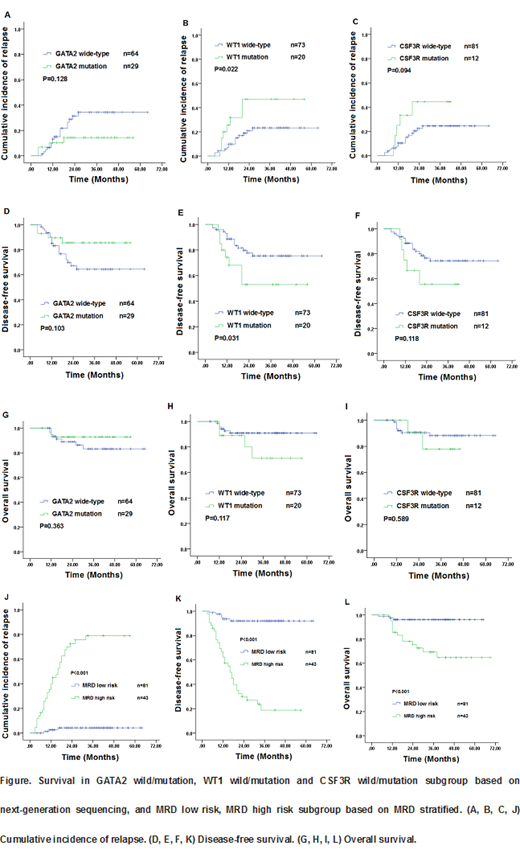
Objectives: Acute myeloid leukemia (AML) patients with biallelic mutations of CEBPA (bi CEBPA) have a 30-50% relapse rate after standard chemotherapy. This study established the value of multiparameter flow cytometric measurable residual disease (MFC-MRD) detection, mutations based on next-generation sequencing (NGS), and compares the outcomes of risk stratification treatment in adult bi CEBPA AML patients. Methods: From March 2014 to December 2018, 124 patients with newly diagnosed acute myeloid leukemia with bi CEBPA were treated with chemotherapy. Out of these, 93 patients were analyzed further by a sensitive NGS assay for mutations in 87 candidate genes. MRD was detected in all patients by MFC after each cycle of induction and consolidation chemotherapy and every 3 months later. Results: Among these patients, 73 patients were male (58.9%), and median age was 37.5 (16-69) years. The expression of CD34, CD117, CD7, HLA-DR, CD15, CD33 and CD13 in 124 patients were 120 (96.8%), 123 (99.2%), 112 (90.3%), 107 (86.3), 93 (75.0%), 115 (92.7%) and 107 (86.3%) respectively. The most common co-occurring mutations were found in the GATA2 (n=29/93, 31%), WT1 (n=20/93, 21.7%), NRAS (n = 13/93, 14.0%), CSF3R (n = 12/93, 12.9%), and TET2 (n = 9/93, 9.6%) genes. All patients (100%) achieved complete remission (CR) including 114 patients (91.9%) who achieved it with only 1 induction course. The median follow-up was 33.5 (4-69) months. The 3-year Cumulative incidence of relapse (CIR), disease-free survival (DFS) and overall survival (OS) were 32.8%, 64.7%, and 84.3%, respectively. Univariate analysis results shown that WT1 Wild-type was favorable factor of 3-year CIR (23.3% vs. 47.0%, P=0.022) and 3-year DFS (75.5% vs 53.0%; P=0.031), GATA2 Wild-type had good trend for lower CIR and longer DFS and CSF3R Wild-type had inferior trend for higher CIR and shorter DFS. Patients with sustained positive MRD status after 2 consolidation cycles and MRD negative status loses in any time defined as "MRD high risk" had higher CIR (3-year CIR, 100% vs 25.5%; P<0.001; 81.5% vs 6.4%; P<0.001, respectively) and worse DFS and OS (3-year DFS, 0% vs 71.5%; P<0.001; 16.8% vs 90.2%; P<0.001, respectively; 3-year OS, 39.0% vs 89.8%; P<0.001; 72.2% vs 96.5%; P=0.001, respectively ) than those with persistent negative MRD status that defined as "MRD low risk" (Figure). Multivariate analysis showed that MRD low risk was the only favorable factor of CIR, DFS and OS (CIR: HR=37.3, 95%CI: 8.6-161.3, P<0.001;. DFS: HR=24.6, 95%CI: 7.2-84.0, P<0.001; HR=28.2, 95%CI: 2.1-374.1, P=0.011; respectively). Thirty-two (91.4%) MRD high risk patients (P<0.001) relapsed at a median time of 8 months (range: 3-32 months) include 21 patients of MRD negative status loses in any time and 11 patients with positive MRD status after 2 consolidation cycles. In totally 35 relapsed patients, 3 patients give up, 19 (61.3%) patients achieved CR2 after induction again. 15 CR2 and 4 non-remission patients underwent allo-HSCT achieved superior 3-year OS (83.0% vs 5.1%; p= 0.034; 75.0% vs 0%; p=0.006; respectively) than chemotherapy. To assess the role of risk stratification treatment by transplant or non-transplant, the124 patients were divided into 2 groups by the unique risk factor: MRD high risk group (n=43, 34.7%) and MRD low risk group (n=81, 65.3%). In the MRD low risk group (median follow-up: 35 months; range: 4-65 months), there was no significant difference in probabilities of 3-year OS (92.3% vs. 96.9%, P=0.428) between the transplant and non-transplant cohorts. In the MRD high risk group (median follow-up: 30 months; range: 7-69 months), 3-year OS was significantly better in the transplant cohort than in the non-transplant cohort (84.0% vs. 44.3%, p=0.007).Conclusions: MRD high risk may better predict the outcome rather than mutations based on NGS in AML with bi CEBPA, and allo-HSCT may achieve superior survival in MRD high risk patients.
Key words: Acute myeloid leukemia with biallelic CEBPA mutations; next-generation sequencing; multiparameter flow cytometric measurable residual disease; allogeneic stem cell transplantation; Chemotherapy
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal