Background: Tumor protein 53 (TP53), located on the short arm of chromosome 17, is an important tumor suppressor gene responsible for critical regulatory functions. There is existing controversy regarding the role of allogeneic stem cell transplantation (allo-SCT) in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) harboring a TP53 mutation. While most studies report increased relapse and poor survival after allo-SCT with TP53 mutated AML or MDS, (Bajar et al., JCO 2014; Lindsley et al., 2017) others found no influence of TP53 mutation on allo-SCT outcomes (Aldoss et al., 2017). Notably, previous studies have predominantly evaluated the prognostic impact of TP53 mutation at the time of diagnosis and not serially in response to treatment. In this study, we aim to clarify the prognostic impact of TP53 mutation clearance prior to allo-SCT in patients with TP53 mutated AML or MDS.
Methods: Data was obtained and analyzed on MDS or AML patients who received allo-SCT at the Moffitt Cancer Center (2013-2018) and had presence of TP53 at least once after diagnosis and prior to allo-SCT. TP53 clearance was defined by last next-generation sequencing (NGS) test prior to allo-SCT demonstrating no TP53 mutation with a variant allele frequency (VAF) greater than 5%. We utilized clinical data captured by BMT Research and Analysis Information Network (BRAIN). Univariate and multivariate analyses were conducted using log-rank and Cox regression, respectively. Kaplan-Meier analysis with log-rank test was used to estimate relapse free survival (RFS) and median overall survival (OS) from the time of allo-SCT. Cumulative incidence of relapse (CIR) and non-relapse mortality (NRM) were performed as defined by the Fine and Gray model.
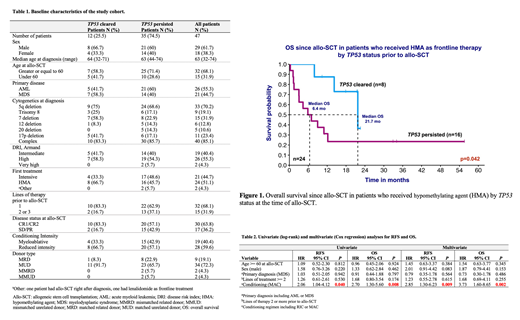
Results: We identified 47 patients (29 males/18 females) with TP53 mutation prior to allo-SCT with primary diagnosis of AML (55.3%) or MDS (44.7%) (Table 1). The median follow-up was 25.4 months with 2-year OS of 32.8% for the entire cohort. The median age at diagnosis was similar between TP53-cleared and TP53-persistent groups with 68% of the patients >= 60 years at the time of allo-SCT. Only 26% (n=12) had clearance of their TP53 mutation prior to allo-SCT. The majority of the patients had complex cytogenetics at diagnosis (85%) with deletion 5q being the most common coexisting aberration (75% in TP53-cleared and 68.6% in TP53-persistent, p=NS). Patients with TP53 clearance received hypomethylating agent (HMA) as frontline treatment more frequently compared to those with persistent TP53 (66.7% vs. 45.7%, p=NS). They also had fewer lines of therapy (83.3% with 1 line vs. 62.9%, p=NS), and a higher rate of complete response prior to allo-SCT (83.3% vs. 57.1%, p=NS). Amongst patients receiving HMA as frontline therapy (n=24), we observed significantly better survival in TP53-cleared patients compared to those with persisted TP53 (median OS of 21.73 months vs. 6.44 months, p=0.042) (Figure 1). Myeloablative conditioning (MAC) regimen was used in 33.3% of TP53-cleared in comparison to 42.9% in TP53-persistent cohort (p=NS). Median OS was 21.7 months for patients with clearance of TP53 vs. 8.1 months for those with persistent TP53 at allo-SCT; although it did not meet statistical significance (p=0.106). MAC compared to reduced intensity conditioning (RIC) regimen resulted in significantly worse RFS (HR 2.06, 95% CI 1.04-4.12, p=0.040) and OS (HR 2.70, 95% CI 1.30-5.60, p=0.008). Conditioning intensity was the only factor that significantly influenced RFS and OS outcomes (Table 2). When repeating these analyses separately on the TP53-cleared and TP53-persistent groups, only the latter remained significant for conditioning (MAC, univariate OS: HR 2.6, 95% CI 1.14-5.92, p=0.023).
Conclusions: TP53 clearance at the time of allo-SCT is predictive of better outcomes in patients who had frontline HMA therapy. For patients with persistent TP53 at the time of allo-SCT, those received MAC experienced worse outcomes compared to RIC in this cohort. The OS at 2-year for TP53-persistent patients is over 30% suggesting even these patients can potentially benefit from transplantation.
Talati:Celgene: Honoraria; Agios: Honoraria; Pfizer: Honoraria; Astellas: Honoraria, Speakers Bureau; Daiichi-Sankyo: Honoraria; Jazz Pharmaceuticals: Honoraria, Speakers Bureau. Sallman:Abbvie: Speakers Bureau; Novartis: Speakers Bureau; Jazz: Research Funding; Incyte: Speakers Bureau; Celyad: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding, Speakers Bureau. Bejanyan:Kiadis Pharma: Other: advisory board. Kuykendall:Celgene: Honoraria; Incyte: Honoraria, Speakers Bureau; Janssen: Consultancy; Abbvie: Honoraria. Padron:Incyte: Research Funding. Komrokji:Incyte: Consultancy; Agios: Consultancy; JAZZ: Consultancy; Novartis: Speakers Bureau; JAZZ: Speakers Bureau; celgene: Consultancy; DSI: Consultancy; pfizer: Consultancy. List:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Lancet:Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Other: fees for non-CME/CE services ; Agios, Biopath, Biosight, Boehringer Inglheim, Celator, Celgene, Janssen, Jazz Pharmaceuticals, Karyopharm, Novartis: Consultancy. Sweet:Incyte: Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau; Stemline: Consultancy; Pfizer: Consultancy; Abbvie: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal