Introduction:
A positive interim PET scan (PET2) following 2 cycles of ABVD for Hodgkin lymphoma (HL) is associated with a poor prognosis; several studies in advanced stage demonstrate benefit from escalating therapy. Definition of positivity (Deauville 3 (D3) vs Deauville 4/5 (D4/5)), initial treatment and response adaptive decisions may vary among clinicians. Data examining practice patterns in managing positive PET2 scans is lacking. We report practice patterns and outcomes for patients (pts) with positive PET2 results including D3 and D4/5 outcomes.
Methods:
Adult pts with classical HL and PET2 findings of D3, D4, and D5 after initial therapy between 01/01/2015 and 07/01/2019 were identified. Pts enrolled in clinical studies were excluded. Retrospective demographic, clinical, and treatment data were obtained and described from 14 academic and community sites across North America (NA). Progression-free survival (PFS) and overall survival (OS) were summarized by Kaplan-Meier curves and compared by log-rank test.
Results:
166 PET2 positive pts were identified. Clinical characteristics included 54% (89/166) with advanced stage (III/IV) and 46% (77/166) with early stage (IA-IIB) disease. 152 pts (92%) were treated with initial ABVD like therapy and 14 pts (8%) with an alternate regimen (Table 1). After initial treatment, 163 pts demonstrated PET2 scores of D3 (n=33), D4 (n=99) and D5 (n=31).
Of the 130 D4/5 pts; 23% (30/130) underwent therapeutic escalation and 77% (100/130) did not escalate. The complete response rate (CR) at end of treatment (EOT) for all D4/5 patients receiving escalation was 37% (11/30) compared to 45% (45/100) without escalation (p=0.43). (Table 2) The 12 month PFS was 54% (38.2-76.3) versus (vs) 69.2% (60.4-79.2) for escalation and no escalation respectively.
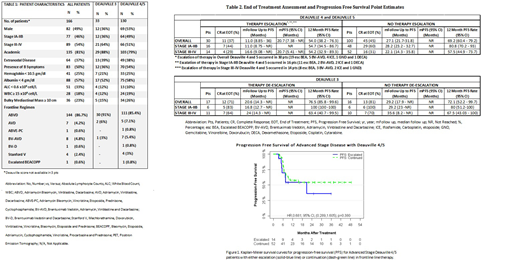
Of the 66 D4/5 pts with advanced stage disease; 21% (14/66) had therapeutic escalation and 79% (52/66) did not escalate. CR at EOT was noted in 29% (4/14) with escalation vs 31% (16/52) without escalation (p=0.88). (Table 2) The 12 month PFS was 54.2% (32.9-89.3) vs 57.5% (44.9-73.7) for escalation and no escalation respectively. (Figure 1; Log Rank p=0.38).
Of the 64 D4/5 pts with early stage disease; 75% (48/64) did not undergo escalation and 25% (16/64) had escalation. CR at EOT was noted in 44% (7/16) with escalation vs 60% (29/48) without escalation (p=0.26). (Table 2) The 12 month PFS was 54.7% (34.5-86.7) vs 80.8% (70.2-92.9) for escalation and no escalation respectively.
Of the 33 D3 pts; 52% (17/33) de-escalated with the remainder (16/33) continuing on initial therapy. No D3 pt underwent escalation. In the 12 D3 pts with early stage disease, 50% (6/12) de-escalated. Of the 21 D3 pts with advanced stage disease; 52% (11/21) had de-escalation and 48% (10/21) did not de-escalate. CR at EOT was noted in 71% (12/17) with de-escalation vs 81% (13/16) without de-escalation (p=0.50). (Table 2)
Progression was noted in 29% (5/17) of patients who underwent de-escalation compared to 38% (6/16) without de-escalation. The 12 month PFS was 76.5% (85.8-99.6) vs 72.1% (52.2-99.7) for de-escalation and no de-escalation respectively.
Conclusion:
This is the first study evaluating clinical practice patterns and outcomes of PET adaptive therapy in NA. In this retrospective study, less than a quarter of advanced stage ABVD-treated D4/5 pts had therapy escalation despite prior studies reporting benefit. Our data suggest that outcomes for advanced stage PET positive pts are suboptimal irrespective of therapeutic escalation. In addition, pts with D3 findings represent a heterogeneous population and demonstrate favorable outcomes compared to D4/5 pts. Longer follow up time and further studies with larger numbers of pts are essential for confirming the reported findings.
Rutherford:Seattle Genetics: Consultancy, Honoraria; Verastem: Consultancy, Honoraria; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Heron: Consultancy, Honoraria; Janssen Scientific Affairs: Consultancy, Honoraria; Juno Therapeutics Inc: Consultancy, Honoraria. Bartlett:ADC Therapeutics: Consultancy, Research Funding; Affimed: Research Funding; Forty Seven: Research Funding; Celgene: Research Funding; Bristol-Myers Squibb: Research Funding; Genenetech: Research Funding; Gilead: Research Funding; Immune Design: Research Funding; Janssen: Research Funding; Merck: Research Funding; Millenium: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Research Funding. Maddocks:Novartis: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; BMS: Research Funding; Teva: Membership on an entity's Board of Directors or advisory committees. Feldman:Takeda: Honoraria, Speakers Bureau; Viracta: Research Funding; Trillium: Research Funding; Roche: Research Funding; Portola Pharma: Research Funding; Pfizer: Research Funding; Kyowa Hakko Kirin: Research Funding; Eisai: Research Funding; Corvus: Research Funding; Roche: Research Funding; Cell Medica: Research Funding; Seattle Genetics: Consultancy, Honoraria, Other: Travel expenses, Speakers Bureau; AbbVie: Honoraria, Other: Travel expenses, Speakers Bureau; Pharmacyclics: Honoraria, Other: Travel expenses, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Kite Pharma: Honoraria, Other: Travel expenses, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Celgene: Honoraria, Research Funding, Speakers Bureau. Magarelli:Tevan Oncology: Speakers Bureau. Advani:Merck: Research Funding; Infinity Pharma: Research Funding; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celmed: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kura: Research Funding; Kyowa Kirin Pharmaceutical Developments, Inc.: Consultancy; Millennium: Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agensys: Research Funding; Seattle Genetics: Consultancy, Research Funding; Stanford University: Employment, Equity Ownership; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Regeneron: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cell Medica, Ltd: Consultancy; Forty-Seven: Research Funding; Autolus: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc./Kite Pharma, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees. Stephens:Karyopharm: Research Funding; Gilead: Research Funding; Acerta: Research Funding. Patel:Sunesis: Consultancy; Genentech: Consultancy, Speakers Bureau; Pharmacyclics/Janssen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau. Tees:Celgene: Speakers Bureau; Pharmacyclics: Speakers Bureau. Karmali:Gilead/Kite; Juno/Celgene: Consultancy, Speakers Bureau; Takeda, BMS: Other: Research Funding to Institution; Astrazeneca: Speakers Bureau. Cheson:Portola: Research Funding; Kite: Research Funding; Gilead: Research Funding; Symbios: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Trillium: Research Funding; Epizyme: Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Yazdy:Bayer: Honoraria, Speakers Bureau; Octapharma: Consultancy; Genentech: Research Funding; Abbvie: Consultancy. Pagel:AstraZeneca: Consultancy; Gilead Sciences: Consultancy; Pharmacyclics: Consultancy. Ramchandren:Seattle Genetics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Merck: Research Funding; Sandoz-Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an Abbvie company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal