Background: Diffuse Large B-cell Lymphoma (DLBCL), the most common lymphoid cancer, is classified by the cell of origin into the germinal and non-germinal center (non-GCB) subtypes. The non-GCB subtype is associated with inferior outcomes with standard therapies, but the BTK inhibitor ibrutinib (I) and immunomodulatory agent lenalidomide (L) have moderate activity as single agents and result in synthetic lethality when combined in non-GCB DLBCL models (Yang, Cancer Cell 2012). When added to chemotherapy for DLBCL as single agents, neither I nor L have significantly improved outcomes over chemotherapy alone as they only result in synthetic lethality with each other. In relapsed non-GCB DLBCL, a phase Ib trial of rituximab (R), L and I resulted in an overall response rate (ORR) of 65% and median duration of response of 15.9 months (Goy, Blood 2019). Both I and L are also immunomodulatory, shifting from a tumor-mediated immune anergy to an anti-tumor immune response.
Methods: We conducted an investigator initiated, open-label, single-arm phase 2 study (Smart Start, NCT02636322) of RLI alone in a 2 cycle lead in followed by RLI combined with standard chemotherapy for 6 cycles in newly diagnosed non-GCB DLBCL patients. Patients were eligible if they had newly diagnosed non-GCB DLBCL (Hans method), adequate organ function and performance status, and were aged 18y or greater. The primary objectives were 1A: to determine the ORR of 2 cycles of RLI, and 1B: to determine the complete response rate (CRR) after RLI-chemotherapy (CHOP or dose adjusted EPOCH per treating MD choice). Therapy consisted of R 375 mg/m2 IV day 1, ibrutinib 560 mg oral daily, and lenalidomide 25 mg oral days 1-10 of a 21 day cycle for 2 cycles, followed by 6 additional cycles of RLI with chemotherapy. All patients were required to receive growth factor support, and prophylaxis for venous thromboembolism and pneumocystis. Responses were determined with PET/CT as per the Lugano criteria.
Results: The protocol accrued 60 patients from May 2016 - February 2019, with 58 patients evaluable for disease response (2 withdrew consent prior to restaging). The median age was 63.5 years (range: 29-83), 28% were >= 70 years, and 50% were female. 51.7% had poor risk R-IPI, 65% had advanced stage, 77% had a Ki-67 of >= 80%, and 54% of patients with available tissue were "double expressor" (DE, MYC and BCL2 overexpression via IHC). The median time from diagnosis to therapy was 24 days. The chemotherapy received was CHOP in 43%, (n=25), dose adjusted EPOCH in 55% (n = 32), and none in 2% (n=1). Over 90% of planned ibrutinib and lenalidomide was able to be administered. 13 patients received less than 6 cycles of chemotherapy (C0 = 1, C4 = 4, C5 = 8) due to toxicity, treating physician preference, or patient refusal.
Adverse events were generally similar to what is expected with chemotherapy, with the exception of rash in 32% (9% grade 3) and CNS aspergillosis (n = 1). 2 patients suffered grade 5 events (CNS aspergillosis, clostridium difficile colitis).
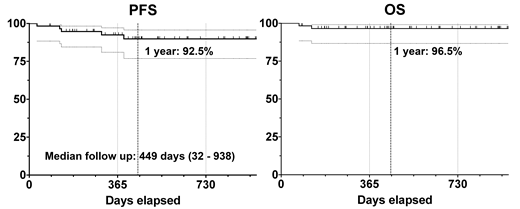
The ORR for 2 cycles of RLI alone was 86% (n=50), the CRR was 36% (n=21), and patients achieving a PR had an 81% median tumor reduction from baseline. The end of therapy ORR is 100% (CR: 95%, PR 5%), with none of the PR patients having relapsed to date without further therapy. The median follow-up at abstract submission is 16 months (1 - 33.5m), with 1 year PFS estimate of 92.5% and OS estimate of 96.5%. Response and survival were not different based upon chemotherapy selected, baseline clinical or pathological variables including DE status or Ki-67%, or number of cycles of therapy received.
Correlative studies, including circulating tumor DNA assessment at baseline, after 2 cycles of RLI, and at the end of therapy and beyond are ongoing and will be presented at the meeting.
Conclusions: The Smart Start trial demonstrates the combination of rituximab 375 mg/m2 IV, ibrutinib 560 mg oral daily, and lenalidomide 25mg oral d1-10 prior to and with chemotherapy results in impressive response rates and survival in newly diagnosed non-GCB DLBCL compared with historical outcomes. Established adverse prognostic variables including non-GCB, high Ki-67%, and double expression of MYC and BCL2 had similar outcomes with other patients with RLI-based therapy. Further studies are planned to expand the lead-in phase with more targeted agents and number of cycles, and to administer fewer cycles of chemotherapy for patients achieving a CR with a targeted therapy lead-in.
Westin:Curis: Other: Advisory Board, Research Funding; Celgene: Other: Advisory Board, Research Funding; Janssen: Other: Advisory Board, Research Funding; Novartis: Other: Advisory Board, Research Funding; Genentech: Other: Advisory Board, Research Funding; 47 Inc: Research Funding; Juno: Other: Advisory Board; Unum: Research Funding; MorphoSys: Other: Advisory Board; Kite: Other: Advisory Board, Research Funding. Nastoupil:Bayer: Honoraria; Celgene: Honoraria, Research Funding; Gilead: Honoraria; Janssen: Honoraria, Research Funding; Spectrum: Honoraria; TG Therapeutics: Honoraria, Research Funding; Novartis: Honoraria; Genentech, Inc.: Honoraria, Research Funding. Oki:Jazz: Employment. Parmar:Cellenkos Inc.: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding. Chuang:Sage Evidence-based Medicine & Practice Institute: Consultancy. Neelapu:Celgene: Consultancy, Research Funding; Unum Therapeutics: Consultancy, Research Funding; BMS: Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Cellectis: Research Funding; Karus: Research Funding; Novartis: Consultancy; Merck: Consultancy, Research Funding; Acerta: Research Funding; Precision Biosciences: Consultancy; Cell Medica: Consultancy; Allogene: Consultancy; Incyte: Consultancy; Poseida: Research Funding; Pfizer: Consultancy.
Ibrutinib and lenalidomide are not yet indicated for DLBCL, we will describe our trial
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal