BACKGROUND: Anti-BCMA CAR T cells are effective in relapsed/refractory multiple myeloma (RRMM), but most patients eventually progress. Extensive prior therapy and high disease burden in RRMM pts may compromise CAR T cell safety, feasibility, and efficacy, and rare BCMA-neg/CD19+ MM cells with enhanced clonogenic potential may mediate relapse after anti-BCMA CARs. We therefore initiated a trial of anti-BCMA CAR T cells (CART-BCMA) as consolidation of response to prior MM therapy, including high-risk (HR) pts responding to first-line therapy, and combined CART-BCMA with anti-CD19 CAR T cells (CTL119). Both CART-BCMA and CTL119 are 4-1BB/CD3z-based CARs transduced via lentiviral vector. CART-BCMA was previously reported (Cohen et al JCI 2019) and exhibited 64% response rate at 5x108 cell dose following cyclophosphamide (cy) alone as lymphodepleting chemo in RRMM. CTL119 is a humanized version of tisagenlecleucel.
METHODS: To assess safety/feasibility of CART-BCMA + CTL119, Phase A (PhA) is evaluating the combination in pts responding (≥MR) to ≥3rd line therapy (or ≥2nd line if exposed to all major agents). After early PhA results showed no excessive toxicity, Phase B (PhB) began enrolling HR pts (R-ISS 3, complex karyotype, PC leukemia, or <PR or early progression on imid/PI induction) responding to first- or second-line therapy, <1y from diagnosis, and unexposed to cytotoxic chemo (except low-dose cy). PhB patients are randomized to receive CART-BCMA +/- CTL119. Both products are dosed at 5x108 CAR+ cells in 3 divided doses (10%, 30%, 60%) after cy 300 mg/m2 + fludarabine 30 mg/m2 daily x 3d (cy/flu). Pts receive maintenance (maint) lenalidomide or pomalidomide beginning d30 or upon recovery from toxicity. Here we report initial results of 6 PhA and 4 PhB pts.
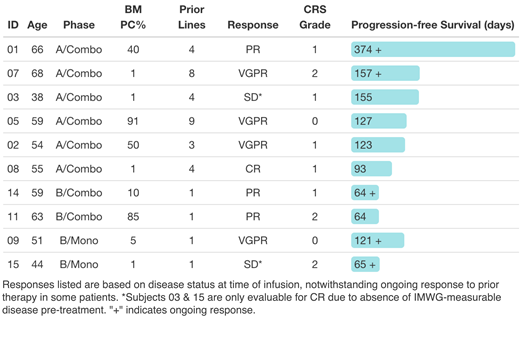
RESULTS: 6/7 enrolled PhA pts were infused; 1 pt died due to CNS progression before infusion. 4/4 enrolled PhB pts were infused (2 CART-BCMA alone, 2 CART-BCMA + CTL119). See table for age & prior # therapies; 9/10 had HR cytogenetics or were R-ISS 3. Regarding mfg feasibility, 2 PhA pts (05, 07) failed to meet full target doses; all 4 PhB pts achieved full dose. Two subjects (01, 03) had the 3rd (60%) dose held due to early fevers. CRS was gr 0-2 by ASTCT criteria and was observed in pts with both high and low disease burden (table). No neurologic toxicity was observed. Gr 3-4 toxicities were mainly hematologic; 3/6 PhA pts had either ANC <1000 and plt <50K at day 30, whereas 4/4 PhB pts recovered ANC & plts by day 30. Aside from CRS, serious AEs ≥possibly related to CAR T cells included pneumonia, sepsis-associated AKI, CMV, pancreatitis, and bone pain, all occurring in PhA pts. Among patients with IMWG-measurable disease, ≥PR was observed in 5/5 PhA pts, including 1 pt with VGPR despite only 10% CART-BCMA dose (pt 05), and 3/3 PhB pts. Responses are ongoing in 2/6 PhA and 3/4 PhB patients (table). Initiation of maint was feasible in 4/6 PhA (median start d67) and 4/4 PhB (median start d48) pts; no recurrence of CRS was observed with maint initiation. All pts exhibited in vivo expansion of both CART19 and CART-BCMA, including those with low disease burden; no re-expansion was observed with maint initiation. Among 5 patients with ongoing responses at day 90 who were evaluable for MRD by flow cytometry, 4 had MRD with detectable BCMA expression (dim in 2 pts, bright in 1 pt, variable in 1 pt), and 1 was MRD-negative (sensitivity ~10-5); 4/5 exhibited absence of normal plasma cells, suggesting possible ongoing anti-BCMA immune surveillance but resistance of residual MM PCs. Among 8 patients who received both CART-BCMA and CTL119 in either PhA or PhB, 5 had ongoing absence of circulating B cells at last follow-up, including two pts with ongoing responses at 1y and 4m, providing evidence for long-term CAR T cell activity in MM patients.
CONCLUSIONS: Preliminary results support safety and high initial response-rate of CART-BCMA + CTL119 after cy/flu in MM. In pts with low disease burden responding to prior therapy, including first-line therapy, CAR T cells expanded in vivo and generated clinical responses with low-grade CRS and no neurotoxicity. Preliminarily, hematologic toxicity and feasibility of maint seem more favorable in first-line setting. Addition of CTL119 did not clearly prevent progression after CART-BCMA in the PhA population (3-9 prior lines); data from PhB with randomization +/- CTL119 are immature. Accrual is ongoing, and updated results will be presented at the meeting.
Garfall:Surface Oncology: Consultancy; Novartis: Patents & Royalties: inventor on patents related to tisagenlecleucel (CTL019) and CART-BCMA, Research Funding; Janssen: Research Funding; Amgen: Research Funding; Tmunity: Honoraria, Research Funding. Cohen:Poseida Therapeutics, Inc.: Research Funding. Lacey:Novartis: Research Funding; Tmunity: Research Funding; Novartis: Patents & Royalties: Patents related to CAR T cell biomarkers. Tian:Novartis: Research Funding. Hwang:Novartis: Research Funding; Tmunity: Research Funding. Vogl:Active Biotech: Consultancy; Janssen: Consultancy; Amgen: Consultancy; Karyopharm Therapeutics: Consultancy; Takeda: Consultancy; Celgene: Consultancy. Lancaster:novartis: Research Funding. Nelson:Novartis: Research Funding. Ferthio:Novartis: Research Funding. Fesnak:Novartis: Research Funding. Melenhorst:National Institutes of Health: Research Funding; IASO Biotherapeutics, Co: Consultancy; Simcere of America, Inc: Consultancy; Incyte: Research Funding; Shanghai Unicar Therapy, Co: Consultancy; Colorado Clinical and Translational Sciences Institute: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding, Speakers Bureau; Stand Up to Cancer: Research Funding; Parker Institute for Cancer Immunotherapy: Research Funding; Genentech: Speakers Bureau. Young:novartis: Research Funding. Levine:Brammer Bio: Membership on an entity's Board of Directors or advisory committees; Vycellix: Membership on an entity's Board of Directors or advisory committees; Incysus: Membership on an entity's Board of Directors or advisory committees; Cure Genetics: Consultancy; Novartis: Consultancy; Novartis: Consultancy, Patents & Royalties, Research Funding; Tmunity Therapeutics: Equity Ownership; CRC Oncology: Consultancy; Avectas: Membership on an entity's Board of Directors or advisory committees. Brogdon:Novartis: Employment. Isaacs:Novartis: Employment. June:Tmunity: Other: scientific founder, for which he has founders stock but no income, Patents & Royalties; Novartis: Research Funding. Milone:Novartis: Patents & Royalties, Research Funding. Stadtmauer:Amgen: Consultancy; Novartis: Consultancy, Research Funding; Tmunity: Research Funding; Abbvie: Research Funding; Celgene: Consultancy; Takeda: Consultancy; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal