Despite intense efforts, multiple myeloma remains incurable in most patients with the standard of care therapies. The plasma cell surface receptor B cell maturation antigen (BMCA) is highly expressed by myeloma cells and we recently demonstrated that 12 out of 25 heavily pretreated myeloma patients achieved a partial response or better after anti-BCMA CAR T cell treatment (VGPR, n=5; CR, n=1; sCR, n=1; Cohen et al., 2019, JCI 129(6):2210). To better understand the biological basis of this therapy, we identified key correlates of response using the pre-manufacturing apheresed T cells, the infusion product, and post-infusion T cells from the 25 patients in this cohort.
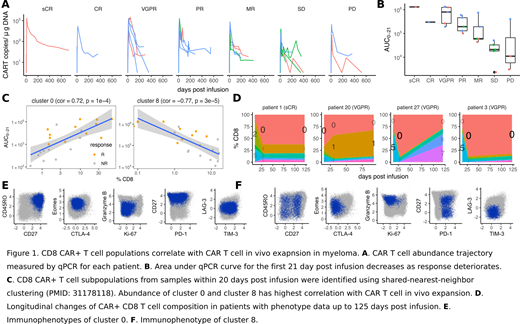
As reported before, the disease characteristics, tumor burden, and CAR transduction efficiency did not correlate with therapy response. CAR T cell expansion, measured by the area under the curve of CAR qPCR in the first 21 days (AUC[0-21]), was highest in responding, lowest in non-responding patients (Jonckheere-Terpstra test, JT = 38, p=1.8x10^-6)(Fig.1A,B). Soluble BCMA, a biomarker of disease burden, shows a similar trend with response (Jonckheere-Terpstra test, JT = 54, p=1.2x10^-4). Furthermore, AUC[0-21] for CAR T cell expansion and soluble BCMA decline also strongly correlated (Spearman's rank correlation test, rho=0.82; p=2.41x10^-6), underscoring the quantitative relationship between CAR T cell expansion and tumor reduction.
We have previously shown that response to CAR T cell therapy in CLL is largely determined by T cell memory function. To find if this extends to myeloma, we immunophenotyped apheresed T cells (or CAR-T precursor cells) and infusion product from the 25 patients. Phenotypically distinct T cell subpopulations were identified using shared-nearest-neighbor clustering method (PMID: 31178118) and their correlation with response to CAR T cell treatment was evaluated. This analysis revealed that among CD4+ and CD8+ CAR-T precursor cells, subpopulations representing naive and central memory T cells were enriched in T cells from responding patients, while non-responders displayed a distinctly activated effector phenotype at baseline. Additional analyses showed that apheresed CD8+ and CD4+ T cells from responder patients were non-cycling, granzyme B-negative, CTLA4[low] but otherwise largely immune checkpoint inhibitor-negative. CD8+ CAR-T precursor cells isolated from non-responders exhibited high expression levels of TIM3 or LAG3, and/or granzyme B, but not PD1, CTLA4, CD45RO or CD27. These data confirm the high activation, potential exhaustion and end-stage differentiation state of CAR-T precursor cells in this group. Similar analyses of infusion product CAR T cells did not reveal subpopulations associated with response.
Clustering analysis of CD8+ CAR T cells within 20 days after infusion revealed a BCMA CAR-expressing cluster enriched in responding patients: a non-cycling, negatively regulated, Eomes-expressing central memory subset (cluster 0; Fig. 1E). Non-responding patients CAR-T cells displayed high levels of granzyme B and PD1 expression but were otherwise devoid of signs of activation (cluster 8; Fig. 1F). Furthermore, the abundance of CD8+ CAR-T cells with cluster 0 and 8 phenotype correlated significantly with in vivo expansion (AUC[0-21]; Fig. 1C). Four patients with a sufficiently high proportion of CAR expressing cells were phenotyped up to 125 days post-infusion. This analysis showed that the highly activated CAR T cell clusters 2 and 5 dominated at early phases post infusion but was rapidly replaced by non-cycling CAR T cells with downregulated CTLA4 and LAG3 but maintained expression of PD1 and TIM3 (cluster 0; Fig. 1D). Patient 27 with VGPR had a prominent effector population four months after infusion. BCMA-redirected CD4+ CAR T cells showed an enrichment of central memory phenotype CAR T cells in responding patients early after infusion, with high expression of Eomes, TIM3, and other immune checkpoint inhibitor molecules. This cluster also dominated the CD4 T cell repertoire in the first four months after infusion in the four responding patients.
In conclusion, our data suggest that strategies to promote expression of Eomes and central memory function and reduce exhaustion in BCMA CAR T cells will enhance clinical activity. Further, these results underscore the "self-sustaining" feature of successful CAR T cell therapies in myeloma.
Pruteanu:Novartis: Employment. Cohen:Poseida Therapeutics, Inc.: Research Funding. Garfall:Tmunity: Honoraria, Research Funding; Amgen: Research Funding; Novartis: Patents & Royalties: inventor on patents related to tisagenlecleucel (CTL019) and CART-BCMA, Research Funding; Janssen: Research Funding; Surface Oncology: Consultancy. Lacey:Novartis: Patents & Royalties: Patents related to CAR T cell biomarkers; Tmunity: Research Funding; Novartis: Research Funding. Fraietta:Tmunity: Research Funding; Cabaletta: Research Funding; LEK Consulting: Consultancy. Brogdon:Novartis: Employment. Davis:Tmunity: Research Funding; Cabaletta: Research Funding. Levine:Tmunity Therapeutics: Equity Ownership; Avectas: Membership on an entity's Board of Directors or advisory committees; Vycellix: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Novartis: Consultancy, Patents & Royalties, Research Funding; Cure Genetics: Consultancy; Incysus: Membership on an entity's Board of Directors or advisory committees; Brammer Bio: Membership on an entity's Board of Directors or advisory committees; CRC Oncology: Consultancy. Milone:Novartis: Research Funding; Novartis: Patents & Royalties: patents related to tisagenlecleucel (CTL019) and CART-BCMA. Stadtmauer:Janssen: Consultancy; Tmunity: Research Funding; Amgen: Consultancy; Abbvie: Research Funding; Novartis: Consultancy, Research Funding; Takeda: Consultancy; Celgene: Consultancy. June:Novartis: Research Funding; Tmunity: Other: scientific founder, for which he has founders stock but no income, Patents & Royalties. Melenhorst:National Institutes of Health: Research Funding; Parker Institute for Cancer Immunotherapy: Research Funding; Novartis: Research Funding, Speakers Bureau; Colorado Clinical and Translational Sciences Institute: Membership on an entity's Board of Directors or advisory committees; Stand Up to Cancer: Research Funding; Incyte: Research Funding; IASO Biotherapeutics, Co: Consultancy; Simcere of America, Inc: Consultancy; Shanghai Unicar Therapy, Co: Consultancy; Genentech: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal