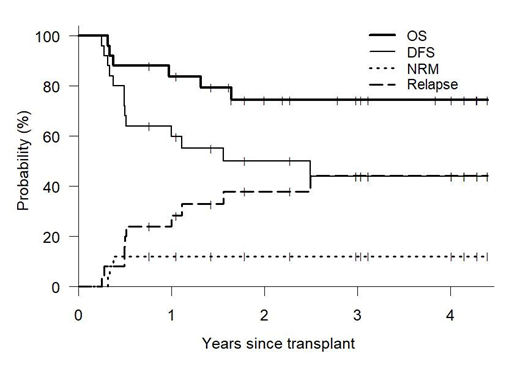
Inhibition of the ubiquitin-proteasome proteolytic pathway results in extensive immunomodulatory effects, augmenting NK cell cytotoxicity while inhibiting aspects of T-, B- and dendritic cell function. To this end, short course bortezomib has been utilized post-transplant to decrease the risk of graft-versus-host disease (GVHD) while preserving graft-versus-malignancy (GVM) effects (Koreth, Haematologica 2018). We performed a phase II study examining the effects of ixazomib for GVHD prophylaxis (up to 12 cycles), in addition to post-transplant cyclophosphamide and tacrolimus, following the original Baltimore nonmyeloablative haploidentical transplant (HIDT) regimen. We hypothesized that substituting ixazomib for mycophenolate would provide acceptable GVHD control while potentially augmenting GVM. Ixazomib was started on day +5 at a dose of 4mg on days 1, 8, 15 of a 28-day cycle, with dose reductions allowed in future cycles for toxicity and mycophenolate, used for conventional post-HIDT GVHD prophylaxis, was eliminated. Donors were selected to maximize NK alloreactivity (i.e. B/x KIR haplotype). Twenty-five patients were enrolled, median age 62 (range 35-77) years, with acute leukemia [4], MDS [7], NHL/HL/CLL [8] and myeloma [6]. Comorbidity index (HCT-CI) was ≥3 in 68%. Donor KIR haplotype was B/x and A/A in 18 and 7 patients respectively. After a median follow-up of 33.5 months (range 9.2-52.8), 19 patients survive with an estimated 1- and 3-year overall survival of 84% and 74% respectively. Engraftment occurred in all patients with no secondary graft failure. Median time to ANC>500 and PLT>20 was 16 and 29 days respectively. CMV reactivation was frequent occurring in 88% of CMV-seropositive patients, however there was no cases of CMV disease. Infectious mortality occurred in 3 patients (disseminated adenovirus, HHV6 encephalitis and fungal pneumonia). The 3-yr cumulative incidence of non-relapse mortality (NRM) was 12% but was significantly higher when the donor possessed the more inhibitory KIR A/A vs. stimulatory B/x haplotype (43% vs. 0%). Relapse incidence at 1- and 3-years was 24% and 44%. Cumulative incidence of Grade 3-4 acute GVHD at day +180 was 8%, while 2-year moderate-to-severe chronic GVHD occurred in 21%. Hematologic and cutaneous toxicities possibly related to ixazomib were common, resulting in dose reductions and treatment interruptions in most patients. Median number of ixazomib cycles was 3 (range 1-12), with only one patient receiving all 12 planned cycles. Rash, at least possibly related to ixazomib, was particularly frequent occurring in 19 (76%) patients at a median day +32 (range, 17-139) following transplant. Reasons for early ixazomib discontinuation included rash [9], cytopenias [7], relapse/progression [4], GVHD [2], diarrhea [1] and arthralgias [1]. In conclusion, the substitution of ixazomib for mycophenolate post-HIDT is feasible and results in reliable engraftment with low rates of clinically significant GVHD and NRM, without any appreciable effect on relapse protection. The occurrence of rash in the first several months in three quarters of patients was unexpected and made it difficult to distinguish drug toxicity from low-grade skin-only acute GVHD. The finding that Infectious mortality occurred exclusively in recipients of KIR A/A haplotype donors is interesting and requires further study.
Solh:Celgene: Speakers Bureau; Amgen: Speakers Bureau; ADC Therapeutics: Research Funding.
Use of ixazomib for GVHD prophlaxis following haploidentical transplantation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal