Introduction: Haplo-cord transplantation - the co-infusion of a single umbilical cord graft with CD34-selected cells from a haplo-identical donor, offers an alternative stem cell source for patients lacking HLA-matched donors. The T-cell depleted haplo-graft acts as a myeloid bridge until replaced by durable cord hematopoiesis.
Anti-thymocyte globulin (ATG) is routinely used in haplo-cord preparative regimens to reduce graft failure, graft vs. host disease (GVHD) and graft vs. graft reactions. ATG depletes T-lymphocytes through Fas-mediated apoptosis, but also indirectly stimulates T-cell destruction through antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC) (de Koning, Blood Adv. 2018). Excessive depletion of cord lymphocytes may compromise immune reconstitution and graft vs. tumor effects.
G-CSF administration early post-transplant may sensitize cord lymphocytes to the cytotoxic effects of residual ATG. G-CSF drives myeloid precursor proliferation and activates phagocytosis, facilitating increased ADCP and ADCC of ATG-coated cells (de Koning, Blood Adv, 2018). Prior to the publication of this data, our institutional practice was to administer G-CSF to haplo-cord transplant recipients from day +6 until neutrophil engraftment. In April 2018, we altered our practice - withholding routine G-CSF treatment. We here compare our transplant outcomes 1 year before and after this practice shift.
Methods: We examined consecutive adult patients with hematologic malignancy that underwent haplo-cord transplantation conditioned with fludarabine and melphalan with or without total body irradiation (400 cGy) at Weill Cornell Medicine, between 04/2017 and 04/2019. All patients received rabbit ATG (Thymoglobulin) 1.5 mg/kg on days -5, -3 and -1 (total dose 4.5 mg/kg). Data was collected as part of study 01810588 registered at clinicaltrials.gov.
Probabilities of relapse, relapse-related mortality (RRM) and non-relapse mortality (NRM) were generated using cumulative incidence (CI) estimates to accommodate competing risks. Probabilities of overall survival (OS) and progression-free survival (PFS) were calculated using Kaplan-Meier estimates and inter-group comparison performed by log-rank testing. Variables with potential survival impact were evaluated in a Cox proportional hazards regression model.
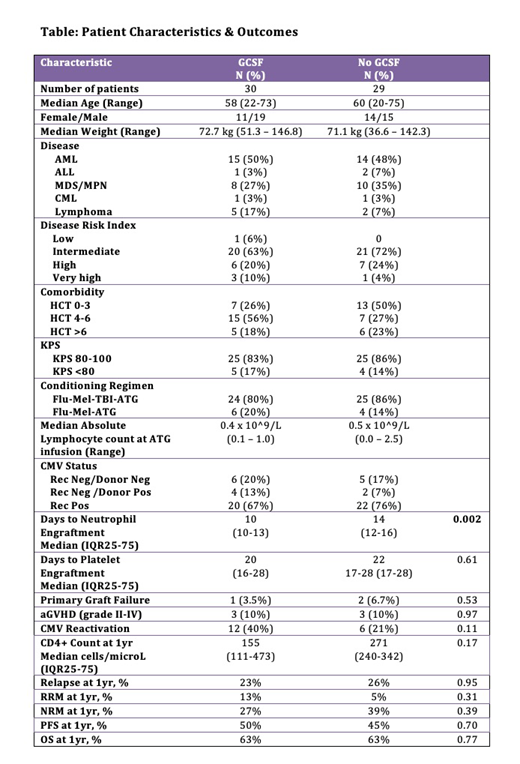
Results: The study included 59 haplo-cord recipients - 30 who received G-CSF (GCSF group) and 29 who did not (NO GCSF group). Groups were well matched for age, weight, disease, DRI, baseline lymphocyte count, CMV recipient/donor status and TBI treatment (Table). Neutrophil engraftment occurred at a median of 10 days in the GCSF group vs. 14 days in the NO GCSF group (p=0.0002), and platelet recovery at a median of 20 and 22 days (p=-0.61). Primary graft failure occurred in 1 GCSF patient and 2 NO GCSF patients (p=0.53).
The incidence of acute GVHD (grade II-IV) by day 100 was 10% in both groups (p=0.97). There was no significant difference between GCSF and NO GCSF in the CIs of relapse, RRM or NRM at 1yr. No difference was found in 1yr OS or PFS on univariate analysis (Table). After adjusting for patient age, weight, lymphocyte count prior to ATG, disease subtype, CIBMTR disease risk index (DRI), Karnofsky performance status (KPS), comorbidity score and TBI exposure, in multivariate analysis, no effect on OS or PFS was observed.
CMV reactivation was observed in 40% (GCSF) vs. 21% (NO GCSF) (p=0.11). The trend towards lower reactivations in the NO GCSF may in part be due to the introduction of letermovir prophylaxis for CMV positive transplant recipients in 02/2018. Acknowledging the limited follow up of NO GCSF patients, we note a trend towards improved CD4 counts at 1yr in this group - median 271 cells/μL (n=6, IQR25-75 = 240-342) vs. 155 cells/μL in the GCSF group (n=16, IQR25-75 = 111-473) (p=0.17).
Conclusion: Our data shows that G-CSF can safely be eliminated from haplo-cord transplant. We see a delay in neutrophil engraftment but no adverse effect on early morbidity or mortality outcomes. We continue to withhold early G-CSF while assessing long-term outcomes. Longer follow-up is needed in our cohort to enable a systematic analysis of immune reconstitution. Early CD4+ cell recovery after ATG treatment has been shown to improve OS, PFS, NRM and RRM (Admiraal, Lancet Hem 2015 & 2017).
Van Besien:Miltenyi Biotec: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal