Introduction: The development of numerous novel therapies for the treatment of relapsed or refractory multiple myeloma (MM) has resulted in improved response rates and durable responses that prolong survival. Assessment of health-related quality of life (HRQoL) has therefore become increasingly important as HRQoL decreases with increasing lines of therapy (LoTs) (Despiégel et al. Clin Lymphoma Myeloma Leuk 2019). In the phase 3 ELOQUENT-2 study (NCT01239797), elotuzumab (E) plus lenalidomide/dexamethasone (Ld) showed a 30% reduction in the risk of progression/death versus Ld in patients with relapsed or refractory MM and 1-3 prior LoTs (median follow-up: 24.5 months; Lonial et al. N Engl J Med 2015). The initial analysis of patient-reported outcomes (PROs) from ELOQUENT-2 at a 3-year extended follow-up showed that the improvement in efficacy observed with ELd was achieved without a detriment to HRQoL (Cella et al. Ann Hematol 2018). Here we present the final analysis of PRO data from ELOQUENT-2.
Methods: In ELOQUENT-2, patients with relapsed or refractory MM and 1-3 prior LoTs were randomized 1:1 to receive ELd or Ld in 28-day cycles until disease progression, unacceptable toxicity, or withdrawal of consent. The Brief Pain Inventory-Short Form (BPI-SF; pain severity, pain interference, and worst pain), the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life-Core 30 questionnaire (QLQ-C30; prespecified key domains were physical function, fatigue, global health status/QoL, and pain), and the myeloma-specific module (QLQ-MY20; includes assessment of symptoms and treatment side effects) were administered at baseline (BL), on Day 1 of each treatment cycle, and at the end of treatment/study withdrawal. All randomized patients with ≥1 post-BL assessment were included in each PRO analysis. Overall mean change from BL was compared between treatment groups based on a mixed-effect model for repeated measures; statistical tests for the overall population only included treatment cycles with >30 patients in each treatment group. A paired t-test was used to compare scores at each cycle with BL; an unpaired t-test compared mean values between treatment groups. BPI-SF scores range from 0-10 with lower scores representing better pain outcomes. EORTC QLQ-C30 scores range from 0-100 with higher scores representing better physical functioning and global health status/QoL, and worse fatigue and pain; EORTC QLQ-MY20 scores range from 0-100 with higher scores representing worse symptoms and problems.
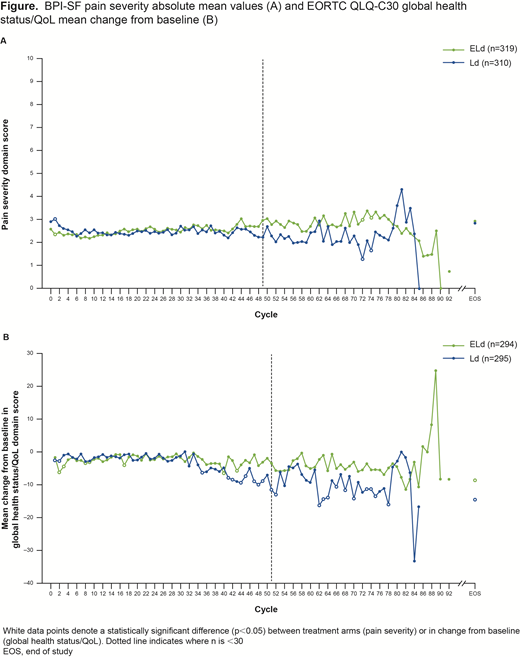
Results: In total, 646 patients were treated with ELd (n=321) or Ld (n=325); 319 and 311 patients had ≥1 post-BL assessment and were included in the PRO analysis, respectively (minimum follow-up: 70.6 months). BL BPI-SF mean scores for ELd versus Ld were low across all domains: pain severity (2.6 vs 2.9), pain interference (2.5 vs 2.8), and worst pain (3.6 vs 3.8). Scores for all BPI-SF domains remained stable over the course of the study (eg, pain severity: Figure A). ELd-treated patients with BL moderate/severe pain severity (score of ≥5) had significantly lower mean pain severity scores versus Ld-treated patients in Cycles 1-5. A higher proportion of clinical responders (complete or partial response per European Group for Blood and Marrow Transplantation criteria) versus non-responders had a sustained reduction in pain score across all BPI-SF domains: pain severity (18% vs 6%), pain interference (15% vs 6%), and worst pain (30% vs 13%); the difference in time to sustained improvement was not statistically significant between the clinical responders and non-responders for any pain endpoint. For both treatment groups, there was no clinically meaningful change (≥10 points) from BL scores at any cycle (>30 patients) across all key domains for EORTC QLQ-C30 (eg, global health status/QoL: Figure B) and QLQ-MY20.
Conclusions: This final analysis of PROs in ELOQUENT-2 confirms that the efficacy benefits observed with addition of elotuzumab to Ld in patients with relapsed or refractory MM treated with 1-3 prior LoTs were achieved without negatively affecting HRQoL compared with Ld.
Study support: BMS. Medical writing: Kenny Tran, Caudex, funded by BMS.
Cella:FACIT.org: Equity Ownership. McKendrick:PRMA Consulting Ltd.: Employment, Other: I am employed by PRMA Consulting Ltd who provide consulting services to a number of pharmaceutical companies. Davis:PRMA Consulting Ltd.: Employment, Other: I am employed by PRMA Consulting Ltd who provide consulting services to a number of pharmaceutical companies.. Vij:Sanofi: Honoraria; Karyopharm: Honoraria; Janssen: Honoraria; Genentech: Honoraria; Celgene: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Takeda: Honoraria, Research Funding. Chen:Bristol-Myers Squibb: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal