Introduction: Hodgkin lymphoma (HL) is one of the most treatable cancers affecting adolescent and young adult (AYA) patients (15 - 39 years), however optimal therapy for de novo disease in this population remains a subject of debate. Population-based studies in HL consistently report a survival disadvantage for AYAs when compared with younger patients. Though the etiology of these disparities is unclear, analyses of clinical trials data suggest that observed survival differences may relate to treatment, rather than to age. Because registry analyses are often limited by lack of information about clinical characteristics and therapeutic exposures, the independent effect of age on HL-outcome outside of the cooperative group setting is unknown. To address this gap in the literature, we: (1) examined initial treatment regimen and patterns of care in a population-based cohort of AYAs compared to children with de novo HL, and (2) examined the impact of sociodemographic and clinical variables on overall survival (OS) and disease-specific survival (DSS) by age, after adjusting for therapy.
Methods: Data for 4,426 patients aged 0 - 39 years diagnosed with classical HL between 2007 and 2016 were obtained from the California Cancer Registry (CCR). Detailed treatment information for each patient was extracted from unstructured free-text fields in the CCR database. Chemotherapy regimens were classified based on standard treatment approaches for adult and pediatric HL (Table). Multivariable cox proportional hazards regression models were used to examine the influence of sociodemographic and clinical variables on OS and DSS, overall and by age group, and are presented as adjusted hazard ratios (aHR) with 95% confidence intervals (CI). Models were adjusted for race/ethnicity, sex, insurance, neighborhood socioeconomic status, histology, stage, B symptoms, treatment location at a NCI (National Cancer Institute)-designated cancer center, and radiation therapy (RT).
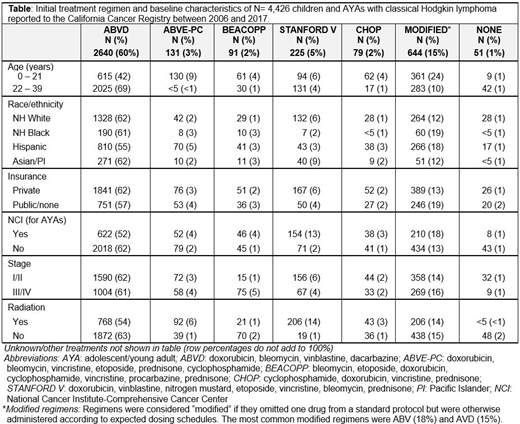
Results: Of the 4,426 patients in this cohort, 33% were <21 years (y) (N= 1,479) and 67% were 22 - 39y (N= 2,947). At median follow-up of 4.4 years, 3-year OS in the full cohort was 95%. Front-line therapy for patients with HL differed significantly across age groups (Table). Approximately 42% of patients <21y received ABVD vs. 69% of older patients. Compared with older patients, a higher proportion of younger patients received ABVE-PC (younger: 8.8% vs. <1%) and modified treatment regimens (younger: 24% vs. 9.6%). Regimens were considered modified if they omitted one drug from a standard protocol but were otherwise administered according to expected dosing schedules; the most common were ABV (18%) and AVD (15%). A higher proportion of patients with private (vs. public/no) insurance received STANFORD V chemotherapy. In total, 40% of patients aged <21y received RT as part of initial therapy vs. 27% of patients 22 - 39y. In survival models, increasing age was associated with a higher risk of death. Compared with patients <14y, the hazard of death from HL was over three-fold higher in patients 22 - 29y (aHR=3.1, CI: 1.1, 9.1) and 30 - 39y (aHR=3.8, CI: 1.3, 11.2). In multivariable models stratified by age, race/ethnicity, insurance, B-symptoms and stage were each significantly associated with survival. In patients <21y, NHBs (aHR: 7.1, CI: 2.4, 20.6) and Hispanics (aHR: 2.5, CI: 1.0, 6.4) experienced worse DSS than NHWs. Having public or no insurance also conferred worse OS (aHR: 1.9, CI: 1.1, 3.5), but initial therapy did not significantly impact OS or DSS. Among those aged 22 - 39y, NHB patients had worse OS (aHR: 1.7, CI: 1.0, 2.8) as did patients with public or no insurance (aHR: 1.7, CI: 1.2, 2.3). Stage IV disease was associated with inferior OS (aHR: 2.9, CI: 1.3, 6.8) and DSS (aHR: 3.3, CI: 1.1, 9.6). Finally, modified treatment regimens (vs. ABVD) were associated with worse OS (aHR: 1.6, CI: 1.0, 2.5), but did not significantly impact DSS in AYAs.
Conclusion: In this large, population-based cohort of children and AYAs with HL, we observed that initial therapy varies, but that the majority of AYAs receive ABVD. Variation in therapy was largely insufficient to explain observed survival disparities, as older age, NHB and Hispanic race/ethnicity, and public or no insurance each conferred increased risk of death, even after adjustment for chemotherapy regimen. Further analyses examining comorbidities, treatment-related toxicities, and cause of death are ongoing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal