Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterised by low platelet counts (<100 x 109/L) and manifests as a bleeding tendency. The demand on hematopoiesis is elevated in chronic ITP, where sustained platelet destruction mediated by an activated immune system is likely to cause considerable stress on progenitor populations. Intriguingly, this increased stress does not appear to result in functional exhaustion, as chronic ITP patients do not present with pancytopenia. By using a novel murine model of chronic ITP, generated by injecting mice with anti-CD41 antibody (ITP group) or IgG (control group) every 48hrs for 4 weeks, we aimed to define the effect of chronic ITP on hematopoietic progenitors and to elucidate the mechanisms behind the preservation of hematopoiesis.
The relative numbers of hematopoietic progenitors in mice with chronic ITP vs controls were analysed by flow cytometry and their fitness was assessed by measuring their relative ability to reconstitute the hematopoietic system of lethally irradiated recipients. There was a significant increase in all hematopoietic progenitors analysed in ITP: 2.96-fold increase in multipotent progenitors, 4.66-fold increase in short-term hematopoietic stem cells (ST-HSCs) and 4.93-fold increase in long-term hematopoietic stem cells (LT-HSCs), which led to an increased ability of ITP donor bone marrow to reconstitute irradiated recipients. The results indicate that chronic ITP drives LT-HSCs out of quiescence and causes increased differentiation into committed progenitors in order to meet the increased demand in platelet production. In support of this, increased megakaryopoiesis was observed in chronic ITP, with a 60.5% increase in the number of megakaryocytes observed in bone marrow sections. Interestingly, similar to the clinical manifestation of ITP, we observed no change in levels of circulating TPO in our ITP model.
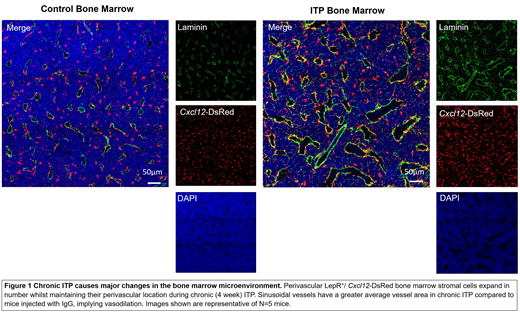
Next, the effect of chronic ITP on the bone marrow microenvironment was determined due to its essential role in the support and maintenance of hematopoiesis. Histological analysis of bone marrow from mice with chronic ITP (Figure 1) revealed a 66.7% increase in the numbers of LepR+/ Cxcl12-DsRed stromal cells. LepR+/ Cxcl12-DsRed stromal cells are a well characterised stromal cell subset, known to be essential for maintenance and retention of HSCs in the bone marrow microenvironment. During chronic ITP, this stromal cell subset maintained their classically defined perivascular location and retained their ability to produce high levels of hematosupportive cytokines (Cxcl12 and Kitl). Chronic ITP was associated with a significant increase in total bone marrow expression (Cxcl12=2.39-fold increase; Kitl=1.71-fold increase), pointing to perivascular stromal cell expansion as being the source of increased local hematopoietic support. Analysis of the bone marrow vascular network revealed that the average vessel area was increased in chronic ITP (54.3% increase), whilst the number of vessels remained unchanged implying that the marrow sinusoids are vasodilated. We hypothesise that an increase in blood vessel area would aid the extravasation of circulating HSCs back into the bone marrow microenvironment where they would contribute to hematopoiesis.
By developing an accurate mouse model of chronic ITP, we have identified key alterations in HSCs and the bone marrow microenvironment. Our data clearly demonstrates that in chronic ITP, HSCs are driven out of quiescence and expand in number in order to contribute to the increased demand for hematopoiesis. Furthermore, the bone marrow microenvironment adapts to this increased differentiation pressure on HSCs by creating a hematosupportive, quiescence promoting environment through the expansion of bone marrow stromal cells, and an increase in blood vessel area.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal