Introduction: BELIEVE is an ongoing phase 3, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of luspatercept, a first-in-class erythroid maturation agent, in adults with β-thalassemia requiring regular RBC transfusions (ClinicalTrials.gov identifier: NCT02604433). Treatment with luspatercept significantly reduced RBC transfusion burden and was associated with a favorable safety profile in this patient (pt) population (Piga A, et al. Blood. 2019;133:1279-89).
Here we report iron-related efficacy endpoints of the BELIEVE study, including change from baseline in serum ferritin, liver iron concentration (LIC), and myocardial iron, as determined by T2*-weighted magnetic resonance imaging (MRI), and the effect of baseline iron levels on response to luspatercept.
Methods: Adults with β-thalassemia or hemoglobin (Hb) E/β-thalassemia who required regular transfusions of 6-20 RBC units in the 24 weeks (wks) prior to randomization (with no transfusion-free period ≥ 35 days during that period) were randomized 2:1 to luspatercept (starting dose level 1.0 mg/kg; titration up to 1.25 mg/kg) or placebo, administered subcutaneously every 3 wks for ≥ 48 wks. Pts in both arms could receive RBC transfusions and iron chelation therapy (ICT) throughout the study to maintain their baseline Hb levels.
Results: 332/336 randomized pts received treatment with luspatercept or placebo; 209/223 (93.7%) and 104/109 (95.4%) pts in the luspatercept and placebo arms had history of iron overload, defined as serum ferritin > 1,000 µg/L or LIC > 7 mg/g dry weight. 97.3% of luspatercept-treated and 97.2% of placebo-treated pts received ICT at baseline; 62.3%, 41.3%, and 37.2% received deferasirox, deferiprone, and deferoxamine, respectively, in the luspatercept arm, vs 57.8%, 36.7%, and 35.8% in the placebo arm. Fewer pts received ICT as combination therapy vs monotherapy (luspatercept arm: 28.6% vs 68.8%; placebo arm: 24.1% vs 72.3%, respectively). Mean baseline serum ferritin, LIC, and myocardial iron (by T2* MRI) for luspatercept vs placebo arms were 2,096.91 vs 1,845.05 μg/L, 12.04 vs 10.09 mg/g, and 33.52 vs 34.76 ms, respectively. As of Jan 7, 2019, 157 (70.4%) continue to receive luspatercept and 92 (84.4%) crossed over from the placebo arm.
During the last 12 wks of the 48-wk randomized treatment period, mean change from baseline in serum ferritin was −248.02 μg/L vs +106.62 μg/L for pts in the luspatercept and placebo arms, respectively (least squares mean [LSM] difference: −347.80; P < 0.0001). 158/224 (70.5%) and 33/112 (29.5%) pts in the luspatercept and placebo treatment arms achieved a ≥ 33% reduction in RBC transfusion burden of ≥ 2 RBC units during any 12-wk interval. Among the responders, mean change from baseline in serum ferritin was −343.22 μg/L and +47.30 μg/L for pts receiving luspatercept and placebo, respectively (LSM difference: −389.50; P = 0.0047). Of 140 luspatercept-treated pts with baseline ferritin ≥ 1,000 μg/L, 29 (20.7%) achieved post baseline ferritin of < 1,000 μg/L when assessed over the entire treatment period; results were similar in responders and non-responders.
During the treatment phase, mean change in myocardial MRI iron signal (T2*) from baseline to Wk 48 was −1.83 ms for luspatercept and +0.02 ms for placebo (LSM difference: −2.39; P = 0.0391). Over 96 wks, 6 (20%) luspatercept-treated pts with baseline myocardial iron ≤ 20 ms had post-baseline results > 20 ms; results were similar between responders and non-responders. Mean LIC changes from baseline to Wk 48 (mg/g dry weight) were +0.10 and +0.08 for luspatercept and placebo arms, respectively (LSM difference: +0.11; P = 0.8685). Over 96 wks, mean change in LIC among luspatercept-treated patients overall was −0.5 mg/g and −1.1 mg/g among responders.
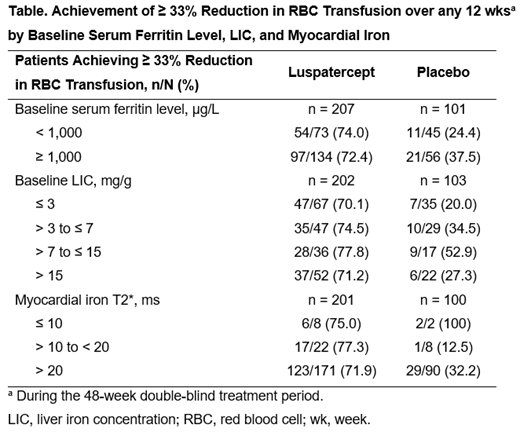
When assessing impact of baseline iron loading there was a general consistency in achievement of ≥ 33% reduction in RBC transfusion with luspatercept over any 12 wks, regardless of iron loading subgroup (baseline serum ferritin, LIC, and myocardial iron) (Table).
Conclusions: Luspatercept treatment resulted in clinically meaningful reductions in serum ferritin levels. Baseline iron overload did not seem to affect response to luspatercept; treatment resulted in clinically meaningful reductions in RBC transfusion burden regardless of baseline serum ferritin level, LIC, or myocardial iron loading.
Porter:Celgene Corporation: Consultancy, Honoraria; Protagonist: Honoraria; Vifor: Honoraria; La Jolla: Honoraria; Agios: Consultancy, Honoraria; Silence Therapeutics: Honoraria; Bluebird Bio: Consultancy, Honoraria. Cappellini:Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Honoraria; Genzyme/Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Vifor Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Membership on an entity's Board of Directors or advisory committees. Coates:apo pharma: Consultancy, Honoraria, Speakers Bureau; agios pharma: Consultancy, Honoraria; celgene: Consultancy, Honoraria, Other: steering committee of clinical study; vifor: Consultancy, Honoraria. Hermine:AB Science: Consultancy, Equity Ownership, Honoraria, Research Funding; Novartis: Research Funding; Celgene: Research Funding. Viprakasit:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene Corporation: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Research Funding; Protagonist: Consultancy, Research Funding; Vifor: Consultancy, Research Funding; Ionis: Consultancy, Research Funding; La Jolla: Consultancy, Research Funding. Voskaridou:Genesis: Consultancy, Research Funding; Protagonist: Research Funding; Celgene Corporation: Consultancy, Research Funding; Acceleron: Consultancy, Research Funding; Addmedica: Membership on an entity's Board of Directors or advisory committees. Perrotta:Acceleron Pharma: Research Funding; Novartis: Honoraria, Research Funding. Kattamis:Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees; Apopharma: Honoraria; Vertex: Membership on an entity's Board of Directors or advisory committees; ViFOR: Membership on an entity's Board of Directors or advisory committees; Ionis: Membership on an entity's Board of Directors or advisory committees; Novartis Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Laadem:Celgene Corporation: Employment, Equity Ownership. Shetty:Celgene Corporation: Employment, Equity Ownership. Kuo:Celgene Corporation: Employment, Equity Ownership. Miteva:Celgene International: Employment. Zinger:Celgene Corporation: Employment. Linde:Acceleron Pharma: Employment, Equity Ownership; Fibrogen, Inc.: Equity Ownership; Abbott Laboratories, Inc.: Equity Ownership. Sinsimer:Celgene Corporation: Employment, Equity Ownership. Taher:Protagonist Therapeutics: Consultancy, Research Funding; La Jolla Pharmaceuticals: Consultancy, Research Funding; Celgene Corporation: Research Funding; Abfero: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Ionis Pharmaceuticals: Consultancy.
Luspatercept is an investigational therapy that is not approved for any use in any country. Luspatercept is currently being evaluated for potential use in patients with anemia due to myelodysplastic syndromes, beta-thalassemia, or myelofibrosis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal