Introduction: Although the most widely-used myeloablative conditioning (MAC) regimen has been a combination of high dose cyclophosphamide (Cy) and total body irradiation (TBI), the optimal radiation dose has not been fully elucidated. While there is evidence that doses exceeding 13 Gy decreases relapse rates, this is associated with excess toxicity. The standard MAC regimen for hematological malignancies at Dana-Farber Cancer Institute prior to 2013 was CyTBI, dosed in seven 2 Gy fractions (14 Gy total). Due to perceived excess toxicity associated with this dose of TBI, from 2013 onward the dose was decreased to 12 Gy (delivered in six, 2 Gy fractions). In the current study, we report our experience comparing outcomes of hematological malignancies, in particular, acute lymphoblastic leukemia (ALL), followed by alloHCT with CyTBI 14 Gy versus 12 Gy in a retrospective analysis.
Methods: We performed a retrospective analysis among patients who underwent an alloHCT at DFCI from January 2010 to December 2017 and who received MAC with CyTBI 14 or 12 Gy as follows: Cy at 1800mg/m2 on days -5 and -4, and TBI on days -3, -2 and -1 (12 Gy total) or with an additional dose on day 0 (14 Gy total). Eligible patients with hematological malignancies met the following criteria: (1) underwent alloHCT with peripheral blood stem cells or bone marrow (2) underwent 8/8 or 7/8 HLA-matched related or unrelated donor alloHCT; and (3) in complete morphologic remission (CR) at the time of transplant. The primary outcome was progression-free survival (PFS) in patients with hematological malignancies with an ALL subset analysis. Secondary outcomes included overall survival (OS), nonrelapse mortality (NRM), relapse incidence (RI), grade II-IV aGVHD and cGVHD in both cohorts.
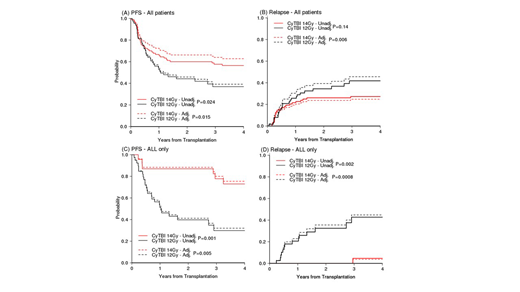
Results: A total of 155 patients met eligibility criteria. Of these, 91 patients received CyTBI 14 Gy (all prior to 2013) while the remaining received CyTBI 12 Gy (n=64) from 2013 onward. Due to the sudden shift of TBI dosing from 14 Gy to 12 Gy and almost exclusive use of TBI in the ALL population only in 2013 forward, the majority (63%) of the 12 Gy cohort consists of ALL patients. 93% in CyTBI 14 Gy and 77% in 14 Gy received peripheral stem cells over bone marrow (P = 0.004). At 3 years, the PFS estimate was 58% (95% CI, 479% to 67%) for CyTBI 14 Gy and 37% (95% CI, 4%- 49%) for those receiving CyTBI 12 Gy conditioning (p=0.024), Figure A - unadjusted curves. In multivariable Cox model adjusting for baseline factors and correcting propensity score, the hazard ratio (HR) of Cy TBI 14Gy to 12 Gy was 0.5 (95% CI 0.29, 0.87, p=0.015) for PFS (adjusted curves in Figure A) and 0.68 (95% CI 0.38, 1.23, p=0.2) for OS. The subdistribution HR (sHR) for relapse from Fine and Gray model was 0.41 (95% CI 0.22, 0.77, p=0.006) (adjusted curves in Figure B) and 0.95 (95% CI 0.34, 2.62, p=0.92) for NRM. The cumulative incidence of Grade II-IV and III-IV aGVHD and cGVHD were not statistically significant (p=0.1, 0.17, and 0.27, respectively) between the two groups. In the planned subset analysis, ALL patients demonstrated a significant superior 3-year PFS (73% vs 30%, respectively, p=0.001) (Figure C - unadjusted curves), OS (82% vs 51%, respectively, p=0.018), and relapse (5% vs 42%, respectively, p=0.002) in the CyTBI 14 Gy cohort compared to 12 Gy. There was no difference in NRM (18% vs 30%, respectively p=0.45). This result was consistent with the result from multivariable models: HR 0.21 (95% CI 0.07, 0.63, p=0.005) for PFS Figure B - adjusted curves, HR 0.31 (95% CI 0.09, 1.03, p=0.056) for OS, sHR 0.04 (95% CI 0.01, 0.25, p=0.0008) for relapse, (Figure D- adjusted curves) and sHR 1.03 (95% CI 0.25, 4.35, p=0.96) for NRM. There was no difference in grade II-IV and III-IV aGVHD in the subset analysis (p=0.55 and 0.49, respectively). However, there was significantly more (p=0.03) cGVHD observed at 2 years in the CyTBI 14 Gy cohort, 78% (95% CI, 53% to 91%) compared to CyTBI 12 Gy, 43% (95% CI, 27% to 58%).
Conclusion: In this single center retrospective analysis of 155 patients with hematological malignancies, PFS and relapse were superior when conditioned with CyTBI 14 compared to 12 Gy. For those diagnosed with ALL, a superior progression free survival and overall survival benefit were more apparent with CyTBI 14 Gy due to significantly less relapses, and thus suggesting consideration of this conditioning regimen for the ALL population.
Ho:Omeros Corporation: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Consultancy. Nikiforow:Kite/Gilead: Honoraria; Novartis: Honoraria; NKarta: Honoraria. Koreth:Amgen: Consultancy; Equillium: Consultancy; Cugene: Consultancy. Ritz:Kite Pharma: Research Funding; Avrobio: Consultancy; Aleta Biotherapeutics: Consultancy; Celgene: Consultancy; Merck: Research Funding; Equillium: Research Funding; TScan Therapeutics: Consultancy; Talaris Therapeutics: Consultancy; Draper Labs: Consultancy; LifeVault Bio: Consultancy. Soiffer:Jazz: Consultancy; Cugene: Consultancy; Mana therapeutic: Consultancy; Gilead, Mana therapeutic, Cugene, Jazz: Consultancy; Juno, kiadis: Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Kiadis: Other: supervisory board. Cutler:Pharmacyclics: Consultancy; Fate Therapeutics: Consultancy; Incyte: Consultancy; Jazz: Consultancy; BMS: Consultancy; Genentech: Consultancy; ElsaLys: Consultancy; Kalytera: Other: DSMB; Cellect: Other: DSMB; BiolineRx: Other: DSMB; Kadmon: Consultancy; Omeros: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal