Background:
Post-transplant lymphoproliferative disorders (PTLD) are a diverse group of lymphoid neoplasms arising after solid organ (SOT) or hematopoietic stem cell transplant (HSCT). They may resemble lymphomas in immunocompetent patients (monomorphic); present as destructive, heterogeneous lesions (polymorphic); or be non-destructive but mass-forming (non-destructive, previously known as 'early lesions').
PTLDs are often associated with Epstein-Barr virus (EBV) infection or reactivation, which likely functions as a driver in these cases. Non-destructive lesions may be challenging to diagnose in the absence of EBV. However, a significant fraction of PTLDs are EBV(-), including a subset of non-destructive lesions. The pathogenesis of these remains to be fully elucidated.
Human herpesvirus 6 (HHV-6) reactivation is common during transplantation, occurring in up to 70% of HSCT and 80% of SOT patients. While it has been hypothesized that HHV-6 may play a role in PTLD, particularly in EBV(-) cases, the role of HHV-6 in the pathogenesis of PTLD remains underexplored.
Methods:
Following IRB approval, pathology archives at Weill Cornell/New York Presbyterian Hospital were searched to identify patients with a tissue diagnosis of PTLD and correlated with clinical information. A tissue microarray was constructed from blocks with sufficient tissue. Standard immunohistochemistry (IHC) and in situ hybridization for EBV-encoded RNA (EBER) were performed, and IHC conditions were optimized for antibodies against HHV-6 gp60/110 (Millipore Sigma, MAB853) and p41 (Santa Cruz Biotechnologies, 9A5D12). Statistical comparisons utilized Student's t-test and Fisher's exact test.
Results:
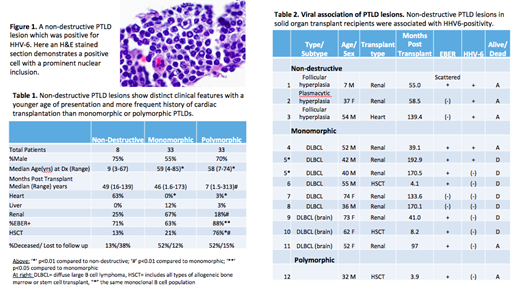
We identified 79 patients from 6/23/94-6/6/19 with a tissue diagnosis of PTLD. Morphologic subtype could be determined in 74, including 8 patients with non-destructive lesions. Compared to monomorphic or polymorphic, patients with non-destructive PTLDs were significantly younger (median age 9, p<0.01) and more likely status post heart transplant (5/8 cases, p<0.001, Table 1). By contrast, polymorphic PTLDs occurred relatively more frequently in HSCT patients (25/33 cases, p<0.01). All morphologic subtypes were frequently EBV+ (63-88%) but EBV positivity was greater in polymorphic than monomorphic lesions (p<0.05).
While tissue testing failed on blocks > 20 years old, 13 PTLD cases from 12 patients had sufficient evaluable material for HHV-6 testing (Table 2). 9/12 patients had a SOT (8 renal, 1 heart) and 3 had HSCT. HHV-6 was identified in 5/13 lesions (38%), all of which were in SOT patients (Figure 1). EBER was detected in 8/13 lesions (62%).
When stratified by PTLD subtype, 3/3 non-destructive PTLDs were HHV-6+ (100%). Two were EBER(-) and the remaining case only showed scattered EBER+ cells. Unfortunately, material was not available for testing the EBER+ non-destructive lesions. 2/9 of the monomorphic lesions tested (22%) were positive for both HHV-6 and EBER. While the series is limited, the association between HHV-6 and non-destructive lesions, compared to other types of PTLD, appears significant (p<0.05). EBV did not appear to be associated with HHV-6 status (p = 0.29).
Conclusions:
Non-destructive, monomorphic and polymorphic PTLDs show significant differences in timing, patient demographics and transplant type, suggesting that they may differ in associated risk factors affecting their pathogenesis. Confirming prior studies, we find non-destructive lesions tend to occur in younger patients, often following SOT as compared to HSCT and with a median time from transplantation of ~50 months. In addition, the presence of HHV-6 in the absence of (or with limited) EBV in the 3 non-destructive lesions tested raises the possibility that HHV-6 may play a role in the pathogenesis of this PTLD subtype that has yet to be defined.
These findings are intriguing given the frequent reactivation of HHV-6 seen in transplant populations. HHV-6 has been variably associated with post-transplant complications including pneumonitis, delayed hematopoietic engraftment and graft versus host disease and thus received relatively more attention in the HSCT population. A causative role for HHV-6 in PTLD remains speculative, but these findings raise the possibility that clinicians treating the SOT population should also consider aggressively identifying and treating HHV-6 reactivation.
Rutherford:AstraZeneca: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Heron: Consultancy, Honoraria; Janssen Scientific Affairs: Consultancy, Honoraria; Juno Therapeutics Inc: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Verastem: Consultancy, Honoraria; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal