Background:
The gain-of function mutation KIT D816V plays a central role in the pathogenesis of systemic mastocytosis (SM). Among subtypes of SM, indolent SM (ISM) is the most frequent and is associated with normal/near-normal life expectancy; smoldering SM (SSM) is a subtype with a relatively higher burden of mast cells and may be associated with an increased risk of progression to advanced mastocytosis. However, both ISM and SSM patients may experience severe symptoms associated with mast cell mediator release, such as pruritus, diarrhea, anaphylaxis, and bone pain, which can be severely debilitating and have a profoundly negative impact on quality of life. Symptomatic treatments (eg, antihistamines and corticosteroids) are used to control symptoms with varying degrees of efficacy; however, these treatments fail to impact mast cell burden, and approved cytoreductive therapies for ISM or SSM are still lacking. There is an urgent unmet need for other treatment options in patients with moderate-to-severe symptoms who do not adequately respond to symptomatic treatment.
Avapritinib is a potent and selective investigational oral kinase inhibitor that targets KIT D816V and other KIT exon 17 mutations. Avapritinib has shown potent and selective in vivo activity against KIT D816V, robust growth inhibition in an in vivo mastocytoma model, and tolerability at active doses in toxicology and safety pharmacology studies. The 3-part, phase 2 PIONEER study is being conducted to identify the recommended phase 2 dose (RP2D) in ISM (part 1), to investigate efficacy of avapritinib vs placebo in patients with ISM and SSM (part 2), and to further characterize the safety and efficacy of long-term treatment with avapritinib (part 3).
Study Design and Methods:
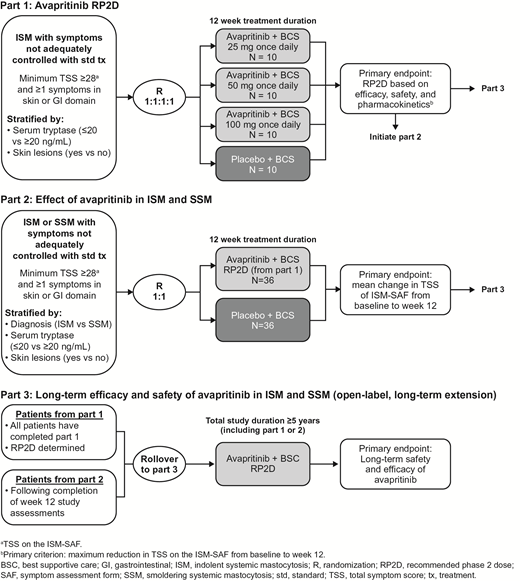
PIONEER (NCT03731260) is an international, multicenter, randomized, double-blind, placebo-controlled phase 2 study of patients with ISM or SSM whose symptoms are not adequately controlled by best supportive care (BSC). For inclusion, patients must have moderate-to-severe symptoms per total symptoms score (TSS) on the ISM-Symptom Assessment Form (SAF) and have failed to achieve symptom control for ≥1 baseline symptom measured by ISM-SAF with ≥2 therapies considered BSC. BSC may include treatments such as H1 and H2 blockers, proton pump inhibitors, corticosteroids, and mast cell stabilizers. The ISM-SAF is being developed specifically to assess symptoms in patients with ISM and SSM, and final validation will be based on data from part 1 of this study.
The study includes 3 parts (Figure). Part 1 aims to identify the RP2D. Patients with ISM will be randomized to three avapritinib doses (25 mg, 50 mg, or 100 mg once daily) or placebo + BSC, stratified by baseline serum tryptase level and the presence/absence of skin lesions. The primary endpoint is R2PD, determined by efficacy, safety, and pharmacokinetics at each dose level, with a primary criterion of maximum reduction in ISM-SAF TSS at week 12. Secondary endpoints include changes in measures of mast cell burden, changes in measures of skin lesions, quality of life, safety, and pharmacokinetics. Part 1 will include approximately 40 patients, with 10 patients per cohort (avapritinib 25, 50, and 100 mg once daily and placebo). Dose investigation will be done in parallel, for a duration of 12 weeks.
In part 2, patients with ISM or SSM will be randomized to the avapritinib RP2D or placebo + BCS to evaluate the efficacy of avapritinib vs placebo in reducing symptoms of ISM and SSM. Patients will be stratified by the same factors as in part 1 as well as by diagnosis (ISM vs SSM). The primary endpoint is mean change in ISM-SAF TSS from baseline to week 12. Secondary endpoints are the same as those in part 1. In part 2, enrollment of 32 patients per group (64 total) will have >90% power to detect superiority of avapritinib to placebo at reducing SM symptoms based on the difference in mean change in the ISM-SAF TSS at week 12 using a 2-sample t-test with a 1-sided type I error rate of 0.025. To account for possible missing data, 35 patients per group will be enrolled (72 total).
Part 3 will evaluate long-term safety and efficacy of avapritinib in ISM and SSM in an open-label extension including patients who completed parts 1 or 2. The primary endpoint is long-term safety and efficacy, secondary objectives include change in TSS ISM-SAF and are similar to those evaluated in parts 1 and 2.
Akin:University of Michigan: Employment; Michigan Allergy and Asthma Society: Membership on an entity's Board of Directors or advisory committees; ECNM: Membership on an entity's Board of Directors or advisory committees; Blueprint: Consultancy, Research Funding; Up to Date: Patents & Royalties; LAD2 cell line: Patents & Royalties; Novartis: Consultancy; NIH: Patents & Royalties. Gotlib:Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Promedior: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Allakos: Honoraria, Membership on an entity's Board of Directors or advisory committees; Deceiphera: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Research Funding. Deininger:Blueprint: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Research Funding; Ascentage Pharma: Consultancy, Honoraria; Novartis: Honoraria; Sangamo: Consultancy; Humana: Honoraria; Incyte: Honoraria; TRM: Consultancy; Sangoma: Consultancy; Fusion Pharma: Consultancy; Adelphi: Consultancy; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Heaney:Partner Therapeutics: Consultancy; Deciphera: Research Funding; Incyte: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Blueprint: Research Funding; Celgene: Research Funding; CTI: Research Funding; BMS: Research Funding; Constellation: Research Funding; Novartis: Consultancy; AbbVie: Consultancy. van Daele:Erasmus MC, Rotterdam: Employment; Novartis: Speakers Bureau. Radia:Blueprint Medicines: Consultancy; Novartis: Consultancy, Speakers Bureau. Triggiani:Novartis: Membership on an entity's Board of Directors or advisory committees; Deciphera: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees. DeAngelo:Amgen, Autolus, Celgene, Forty-seven, Incyte, Jazzs, Pfizer, Shire, Takeda: Consultancy; Blueprint: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Abbvie: Research Funding; Glycomimetics: Research Funding. George:Blueprint Medicines: Consultancy; Deciphera: Consultancy; Novartis: Honoraria; Allakos: Consultancy. Hartmann:ALK-Abello: Consultancy; Bluepriont: Consultancy; Deciphera: Consultancy; Novartis: Consultancy; Euroimmun: Research Funding. Frank:Blueprint: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Allakos: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Reiter:Novartis: Consultancy, Honoraria, Other: Travel reimbursement, Research Funding; Blueprint: Consultancy, Honoraria, Other: Travel reimbursement; Deciphera: Consultancy, Honoraria, Other: Travel reimbursement. Panse:Roche: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Chugai: Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees. Thaci:AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Medimmune: Honoraria; Boehringer Ingelheim: Consultancy; Morphosis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Speakers Bureau; Galderma: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Leo Pharma: Consultancy, Honoraria, Speakers Bureau; DS-Biopharma: Consultancy, Honoraria; UCB: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Glaxo-Smith Kline: Consultancy, Honoraria, Speakers Bureau; Samsung: Consultancy, Honoraria; Lilly: Consultancy, Honoraria, Speakers Bureau; Almiral: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Merck Sharp & Dohme: Honoraria; Sandoz: Consultancy, Honoraria, Speakers Bureau; Regeneron: Consultancy, Honoraria; Medac: Consultancy, Honoraria, Speakers Bureau. Lin:Blueprint Medicines: Employment. Morrison:Blueprint Medicines: Employment. Mar:Blueprint Medicines: Employment. Maurer:Blueprint: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Allakos: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal