Background: Graft-versus-host disease (GVHD) is a dreaded complication after stem-cell transplantation (SCT). Standard treatment relies on immunosuppressants but is associated with an increased risk of infection and relapse. Up 50% of patients develop steroid-refractory GVHD, with dismal impact on SCT outcomes.
New studies in mice suggest that induction of type-I interferon (IFN-I) signalling or activation of IFN-I inducing pathways such as cGAS/STING or RIG-I/MAVS can promote gut barrier integrity and limit GVHD. However, the endogenous ligands that drive this "protective" IFN-I response are poorly defined. Recent data in mice and humans suggest that microbial-derived metabolites such as small-chain fatty acids or indoles can decrease GHVD mortality. Here, we describe IFN-I inducing metabolites that improve outcomes in mouse models of gut epithelial damage and acute GVHD via a mechanism dependent on STING signalling.
Methods: To investigate which cell types mediate protection and how, we generated intestinal organoids and antigen presenting cells (APC) from bone marrow of WT or genetically deficient mice (MAVS-/-, STING-/-, IFNAR-/-) under steady state conditions versus chemotherapy, total body irradiation and after allogeneic SCT in the presence or absence of microbial metabolites. Analysis was performed by microscopy, immunoblotting, qPCR, ELISA and flow cytometry. Outcomes of gut-injured mice were assessed by clinical scoring, histopathology, flow cytometry and organoid recovery.
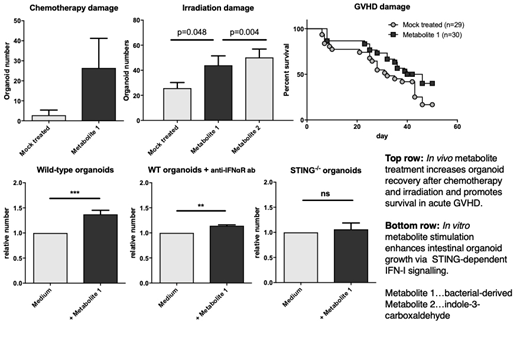
Results: Metabolite treatment promoted regeneration of intestinal organoids as assayed by organoid numbers as well as proliferation. These effects were dependent on IFN-I and STING signalling. In addition, we found activated pro-inflammatory NFkB signalling and decreased apoptosis as evidenced by reduced caspase-3 cleavage. In APCs, IFN-I responses were enhanced in the presence of metabolites including increased IFN-β production and upregulation of IFN stimulated genes. Metabolite-treated mice showed improved recovery of intestinal stem cells following gut injury by chemotherapy and irradiation and increased survival in acute GVHD.
Conclusions: Our findings uncover a mechanism by which microbial metabolites amplify IFN-I signals, promote tissue regeneration and thereby prevent allo-activation and GVHD. We show for the first time that these IFN-I inducing metabolites are protective in diverse models of gut damage -- from chemotherapy and radiation to immune-mediated damage -- via a STING-dependent mechanism. Perhaps the poor prognosis of GVHD patients exhibiting a loss of microbiota diversity can be explained in part by an absence of "protective" metabolites able to amplify critical IFN signals. We are currently studying whether metabolite levels correlate with severity and outcome of GVHD in humans.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal