Introduction
Chronic graft-versus-host disease (cGVHD) is an autoimmune-like condition caused by allogeneic hematopoietic stem cell transplantation. It is the major cause of non-relapse mortality and morbidity after transplant. The majority of patients with cGVHD experience longstanding fatigue that is associated with decreased quality of life and impaired physical functioning. The pathophysiology and clinical characteristics of fatigue in cGVHD remain poorly understood and no effective treatments exist.
Methods
This cross-sectional study aimed to describe characteristics of fatigue experienced by patients with cGVHD using SF-36 self-report vitality scale as the primary outcome. 109 patients with fatigue and 72 patients without fatigue were prospectively enrolled and compared using a set of clinical, demographic and behavioral data along with 7 potential cytokine biomarkers of active cGVHD (IFN- γ, IL-6, CXCL-10, MCP-1, CXCL-9, BAFF, ST2). IFN- γ, IL-6, IP-10, and MCP-1 were assayed using V-PLEX by from Meso-ScaleDiscovery (MSD). The BAFF, CXCL9, and ST2 high sensitive assays were developed and customized for clinical testing using MSD electrochemiluminescence immunoassay technology with antibody pairs obtained from R&D Systems. A patient was considered fatigued if SF-36 vitality score was >10 points below the mean of the general population (<40).
Results
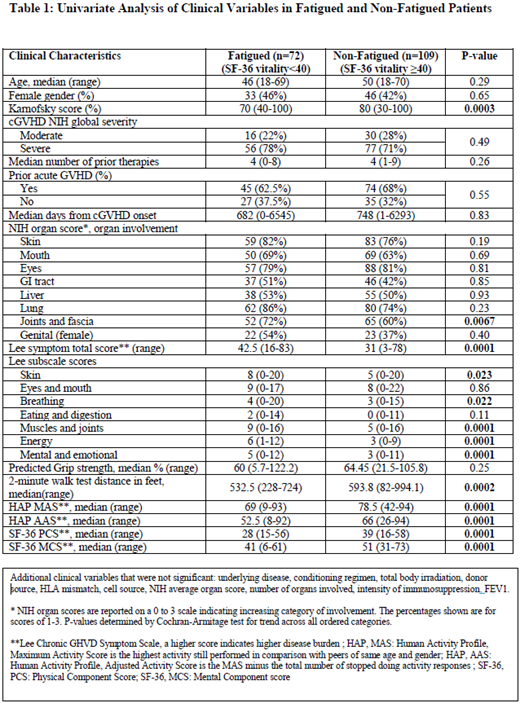
The median Lee cGVHD symptom scale energy score was 6 (1-12) in fatigued individuals as compared to 3 (0-9) in non-fatigued individuals (P=0.0001) suggesting internal validity of the primary endpoint definition. SF-36 MCS and PCS scores were also significantly lower in the fatigued group by 10 points (P=0.0001 for both). Several measures including walk velocity, human activity profile, SF-36 physical and mental health self-report scales were potential predictors of fatigue in univariate analysis (Table 1). Patients with increasing levels of joint involvement were more likely to be fatigued (72% vs 60% with any involvement, p= 0.0067 for increasing involvement score). In addition, fatigued individuals had higher Lee cGVHD symptom burden in skin, breathing, muscles and joints and mental domains. None of the tested cytokines showed significant association with fatigue, even after stratification for time elapsed after cGVHD diagnosis to study enrollment. However, BAFF was shown to decrease in a consistent manner when more time elapsed from cGVHD diagnosis to study enrolment (p=0.015). BAFF levels were significantly associated with NIH joint score, NIH skin score, intensity of immunosuppression and Karnofsky performance status, thus confirming the usefulness of BAFF as a marker of cGVHD activity or severity, but not fatigue. Similarly, none of standard clinical laboratory serum markers of inflammation (e.g. ESR, CRP, C3, C4, albumin) showed significant association with fatigue.
Multivariable analysis revealed that NIH joint score (P=0.03), Lee symptom scale sleep (P=0.0004) and depression (P=0.03) questions and PG-SGA nutrition score (P=0.0007) were predictors of fatigue after adjusting for confounders. Antidepressant use or sleep medication use did not differ significantly between the 2 groups (p=0.63 and 0.51, respectively), although patients reporting depression and sleep problems were more likely to be in the fatigued group, thus delineating a potential area for intervention and increasing quality of life. After adjusting for NIH lung score and performance status, known poor predictors of survival in this patient population, fatigue was associated with a slight trend toward lower probability of survival, median 130.6 months vs. median not reached (p=0.074) for fatigued and non-fatigued respectively (HR: 1.45, CI 0.88 to 2.40).
Conclusions
These results demonstrate that the pathophysiology of fatigue in cGVHD may involve mechanisms other than those reflected by cytokine markers of inflammation known to be associated with disease activity or severity. Musculoskeletal and lung involvement as well as decreased functional performance and capacity are significant features of fatigued patients with cGVHD. Sleep, nutrition and depression may be important therapeutic targets in these patients affected by clinically significant fatigue. Further studies involving longitudinal follow-up and deeper studies of biology are imperative to better understand or treat fatigue in cGVHD patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal