Background
The treatment algorithm is still limited in WM as very few drugs were approved based on studies dedicated to WM patients. In 2015, the Bruton tyrosine kinase (BTK) inhibitor ibrutinib became the first drug approved to treat WM (Treon et al, 2015). However, a subset of patients (pts) relapses due to acquired resistance. Therefore there is a great medical need to develop chemo-free approaches based on a better understanding of the biology of the disease to increase all survival endpoints. MYD88L265P also promotes activation of the phosphatidylinositol-3-kinase (PI3K) pathway Gopal et al. reported 80% ORR in 10 patients with WM, refractory to anti-CD20 and alkylating agents, treated with idelalisib (a PI3K inhibitor) (NEJM, 2014). A previous study was stopped because of high incidence of hepatotoxicity (Castillo, Leuk lymphoma, 2017). So we design our trial with a Bayesian analysis of adverse events.
Aims
We initiated a prospective, single-arm phase II study to evaluate efficacy and safety of idelalisib in combination with obinutuzumab in pts with R/R WM in need of treatment. (NCT02962401).
Methods
During the induction phase, idelalisib was given continuously 150 mg BID PO in association with IV obinutuzumab 100mg day 1, 900mg day 2 then 1000mg fixed dose day 8, 15 of cycle 1 and every day
1 of cycles 2 to 6 (28-days cycle). Then during the maintenance phase, idelalisib was given alone for a maximum of 2 years. Pts were closely monitored for infusion related reactions (IRR). Adverse events were graded per CTCAE v.4.0. Response was assessed based on IWWM6 criteria. The analyses of PFS, primary endpoint of this study, were based on the intent-to-treat population. The safety analysis was designed according to Bayesian estimation of the probability of grade 3 or more adverse events. Roche and Gilead provide drugs and funding.
Results
Fifty pts were enrolled between February 2017 and July 2018 but 49 pts were analyzed (1 screen failure). We present the results of early efficacy and safety, assessed after the induction phase. At time of analysis, median follow-up was 18.3 months (range 14.9-23 months). Median age was 71 years (range 50-83 years) and 36 pts (73%) were men. MYD88 mutation was present in 47 pts (96%). Indications to treat were anemia (31%), anemia + thombocytopenia (11%), constitutional symptoms (11%), rapid evolution of monoclonal component (11%), hyperviscosity syndrome (9%), thrombocytopenia (6%), extramedullary disease (8%) and neuropathy (6%) (data not available for 2 pts). Median number of previous lines of therapy was 1 (range 1-3), and only 1 patient was previously exposed to BTK inhibitors. At baseline, median serum IgM was 2.193 mg/dl (range 0.19-9.2), median bone marrow involvement was 55% (range 10-90, n=25) and median hemoglobin was 10 g/dl (range 6.5-13.8).
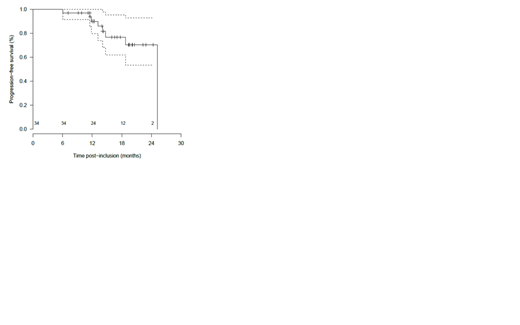
Thirty-four pts responded, 13 after 3 cycles and 21 after 6 cycles. The overall response rate (ORR) was 90% and the major response rate (MRR) was 76% (no CR, VGPR: 8%, PR: 68%, MR: 14%, SD: 8%, and progression: 3%). The correlation between responses and genomic status using ultra deep next generation sequencing will be communicated later. Median PFS was 25.2 months. The1-year and 2-year PFS were 90% [IC95%: 80; 100] and 70% [IC95%: 53; 93] respectively. The1-year and 2-year OS were 98% [IC95%: 94; 100] and 85% [IC95%: 69; 100] respectively. Three pts died (1 before starting treatment, 1 from macrophage activation syndrome, 1 after stopping treatment). Median duration of response was 21.8 months. No flare, no IRR grade ≥ 2, no tumor lysis syndrome were observed. Thirty-five pts experienced at least one grade ≥ 3 adverse events (AE) or serious adverse events (SAE), with an estimated probability of 72.5% (95% credibility interval, 59.5-83.9) and a probability of 1 that more than 30% of pts experience at least 1 grade 3 AE. Events that occurred most frequently included hepatotoxicity (23 AE, 5 SAE, 20%), diarrhea (4 AE, 10 SAE; 10%), skin (3 AE, 3 SAE, 4%), infections (0 AE, 5 SAE, 4%), neutropenia (41 AE, 29%), anemia (7 AE, 5%), thrombopenia (6 AE, 4%). This trial is ongoing with 29 pts who started idelalisib maintenance.
Conclusion
This is the first study evaluating combination with idelalisib + obinutuzumab and the first chemo-free fixed-duration association in R/R WM pts. The combination has clinical activity with 90% ORR and 76% MRR. Median PFS was 25 months. Most of grade ≥ 3 AE or SAE are hepatotoxicity, diarrhea and neutropenia as expected with idelalisib.
Tomowiak:Abbvie: Honoraria; Janssen: Honoraria. Perrot:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; Amgen: Honoraria; takeda: Honoraria; jannsen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Leblond:Gilead: Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal