Introduction: Adults with (w/) B-cell acute lymphoblastic leukemia (B-ALL) exhibit high rates of complete response (CR) to induction chemotherapy, but relapse is common. Inotuzumab ozogamicin (IO), an antibody-drug conjugate targeting CD22, achieves high rates of CR in patients (pts) w/ relapsed/refractory (R/R) B-ALL and is FDA-approved for R/R B-ALL in adults. It remains unknown whether cytogenetic and molecular features associated w/ decreased response rate and poor prognosis following conventional chemotherapy are associated w/ response to IO. As such, we investigated the relationship between several high-risk genetic alterations and outcome following IO treatment in pts w/ R/R B-ALL.
Methods: We reviewed electronic medical records of pts of all ages w/ R/R B-ALL or chronic myeloid leukemia in lymphoid blast phase (CML-LBP) receiving IO at Memorial Sloan Kettering Cancer Center (MSK) between January 2011 and April 2019. The primary objective was to assess whether recurrent cytogenetic or molecular features were associated w/ achievement of CR or CR w/ incomplete hematologic recovery (CRi), w/ or w/o measurable residual disease (MRD), and disease-free (DFS) and overall survival (OS) following IO. Secondary objectives included association of baseline clinical features, including central nervous system (CNS) or other extramedullary (EM) disease, w/ outcomes post-IO. MRD was defined as any unequivocal evidence of B-ALL detectable by RT-PCR (Ph+ ALL) or flow cytometry (FACS). Genomic alterations were defined by MSK IMPACT-Heme (Cheng, J Mol Diagn, 2015), FoundationOne Heme, or similar platforms. A set of selected high-risk (HR) features in Philadelphia chromosome-negative (Ph-) B-ALL was defined prior to the analysis (HR: mutations/loss of TP53, IKZF1/3, CDKN2A, CREBBP; activating RAS mutations; "Ph-like" profile). DFS and OS were computed using Kaplan-Meier methods and compared between groups using log-tank tests.
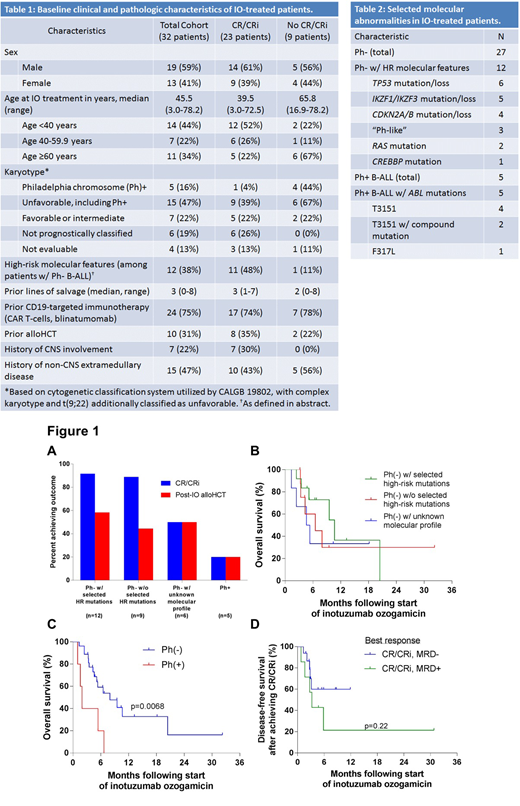
Results: 32 pts (13F, 19M) w/ R/R B-ALL (n=31) or CML-LBP (n=1) treated w/ IO were identified. IO was given as monotherapy in 27 pts and w/ other systemic therapy in 5 pts (mini-hyper-CVD-like regimen, n=4; ponatinib, n=1). Median age at start of IO was 45 years (range 3-78). 10 pts had undergone prior allogeneic hematopoietic cell transplantation (alloHCT). Seven and 15 pts had a history of CNS disease or other EM involvement by B-ALL, respectively, including 3 and 6 pts immediately prior to IO, respectively. Pts received a median 3 lines of salvage prior to IO, including prior CD19-targeted immunotherapy (blinatumomab and/or CAR-T cells) in 24 pts(Table 1).
Among 27 pts w/ Ph- B-ALL, 12 had the selected HR features (Table 2). Five pts had Ph+ ALL (n=4) or CML-LBP (n=1) and 5/5 harbored ABL1 kinase domain point mutations (4/5 w/ T315I mutation). 22 pts had at least one successful molecular profiling panel.29 patients had initial cytogenetic studies, of whom 28 patients had evaluable karyotypes.
23 pts had best response to IO of CR/CRi (MRD-, n=15; MRD+, n=8). 9 pts had no objective response to ≥1 cycle of IO. Of the 12 Ph- pts w/ selected HR mutations, 11 achieved CR/CRi. Notably, 6/6 pts w/ TP53 mutation/deletion and 5/5 pts w/ IKZF1/3 mutations (3/3 pts w/ both TP53 & IKZF mutations) achieved CR/CRi. Both pts w/ Ras mutations and 2/3 w/ Ph-like B-ALL achieved CR/CRi. 7/11 HR responders underwent alloHCT post-IO (3 had undergone pre-IO alloHCT). Pts w/ Ph- B-ALL w/ HR mutations demonstrated similar CR/CRi rate and OS to pts w/ Ph- B-ALL w/o defined HR mutations (Fig 1A-B). In contrast, only 1/5 pts w/ Ph+ ALL achieved CR/CRi (was MRD+) and 4/5 showed persistent B-ALL. OS was superior among pts w/ Ph- vs Ph+ B-ALL post-IO (8.0 vs 1.9 months, p=0.0068, Fig 1C). Among pts w/ EM disease immediately prior to IO, 3/6 achieved CR/CRi, including CR in 1 pt w/ a cardiac mass. Median DFS was 3.2 months vs. not reached following achievement of MRD+ vs MRD- CR, respectively (p=ns, Fig 1D).
Conclusions: HR molecular features associated w/ poor response to chemotherapy were not associated w/ inferior response rate and overall prognosis following IO in this small series. Notably, pts w/ Ph+ ALL (all w/ ABL1 mutations) exhibited suboptimal response, possibly as pts received IO only in advanced disease states following TKI failure. This small report supports investigation of IO in frontline therapy for pts w/ B-ALL w/ HR mutations to spare unnecessary toxicities of chemotherapy and bridge successfully to alloHCT.
King:Genentech: Other: Advisory Board ; Astrazeneca: Other: Advisory board; Incyte: Other: Advisory Board. Park:Allogene: Consultancy; Amgen: Consultancy; AstraZeneca: Consultancy; Autolus: Consultancy; GSK: Consultancy; Incyte: Consultancy; Kite Pharma: Consultancy; Novartis: Consultancy; Takeda: Consultancy. Geyer:Dava Oncology: Honoraria; Amgen: Research Funding.
Inotuzumab ozogamicin is not FDA approved for pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal