Introduction: Antibody-drug conjugate polatuzumab vedotin (pola) targets CD79b on B-cell non-Hodgkin lymphoma. Cytopenias and peripheral neuropathy (PN) are typical pola-associated adverse events (AEs). We reported significantly higher positron emission tomography-complete response (PET-CR) rates and improved duration of response (DOR), progression-free survival (PFS) and overall survival (OS) with pola + bendamustine-rituximab (BR) vs BR in patients (pts) with R/R DLBCL (Sehn et al. ASH 2018). With an additional year of follow up, we report updated results for the Ph Ib/II DLBCL cohorts (NCT02257567).
Methods: Methods were previously reported (Sehn et al. ASH 2018). Safety-evaluable population combined Ph Ib and randomized (RAN) Ph II pola + BR arms (N=45) vs RAN BR (N=39) arm. Longer-term safety outcomes for PN and second malignancies were evaluated. Efficacy reported separately for Ph Ib/II RAN DLBCL arms per investigator (INV) assessment. Additional outcomes including DOR for pts with confirmed responses (defined as having two consecutive responses) were assessed.
Results: As of March 15 2019, median follow up for pts with R/R DLBCL in the Ph Ib (N=6) and Ph II RAN arms (N=80) was 46 and 30 months (mo), respectively. In the RAN Ph II (pola + BR [N=40]; BR [N=40]; 39 treated per arm), baseline characteristics were largely balanced with median 2 prior lines of therapy; 28% vs 30% of pts had 1 prior line, 28% vs 23% had 2, 45% vs 48% had ≥3 in the pola + BR vs BR arms, respectively. Most pts (75% vs 85%) were refractory to last prior treatment.
PN events occurred in 40% (18/45) of pts treated with pola + BR (all grade [Gr] 1-2). At clinical cutoff, median time to PN resolution was approx. 8 days (range 0-69) with 56% (10/18) of pts experiencing complete resolution of PN. Eight pts had ongoing PN: 4 discontinued study early due to death from disease progression or AE (further follow up for PN resolution not possible), 3 had unresolved Gr 1 PN, and 1 had improving PN (max. Gr 1). Second malignancies occurred in 4% (2/45) of pts treated with pola + BR (1 prostate cancer; 1 squamous cell carcinoma and myelodysplastic syndrome [MDS]) and 5% (2/39) of pts in the BR arm (1 epiglottic carcinoma and MDS; 1 papillary thyroid cancer).
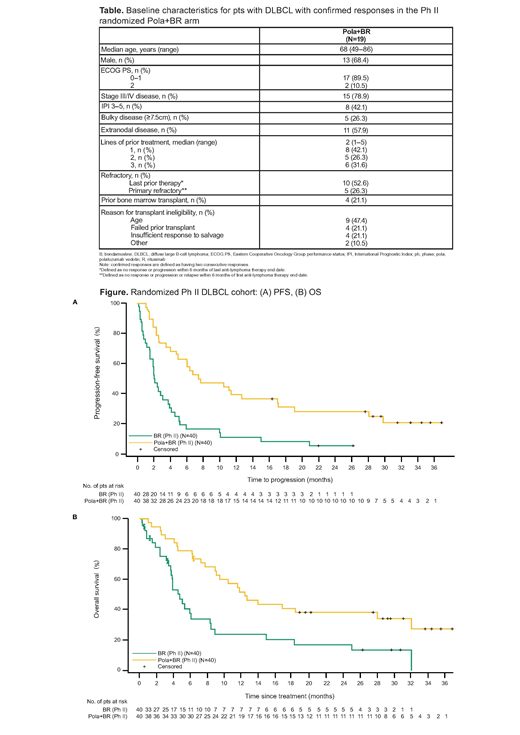
In the Ph II RAN arms (pola + BR vs BR) updated median INV-assessed PFS (95% confidence interval [CI]) was 7.5 (4.9, 17.0) vs 2.0 (1.5, 3.7) mo (hazard ratio [HR] 0.33; 95% CI 0.20, 0.56); median OS (95% CI) was 12.4 (9.0, 32.0) vs 4.7 (3.7, 8.3) mo (HR 0.41; 95% CI 0.24, 0.71), respectively (Figure). Median time to first response was 2 mo (range 1.8-5.3). Median DOR (95% CI) for all responding pts in the pola + BR (N=28) vs BR (N=13) arms was 12.7 (5.8, 27.9) vs 4.1 (2.6, 12.7) mo (HR 0.42; 95% CI 0.19, 0.91). Median DOR (95% CI) for confirmed responders in the pola + BR (N=19) vs BR (N=7) arms was 27.9 (10.3, NE) vs 12.7 (7.7, NE) mo (HR 0.44; 95% CI 0.14, 1.32). Nearly half (47%, 9/19) of pts with confirmed responses to pola + BR remained event free at clinical cutoff with 8 pts in CR and 1 pt in partial response (downgraded from CR due to missing bone marrow biopsy based on modified Lugano Criteria). Of these 9 pts, 8 had DORs ranging from 22+ to 34+ mo (1 pt consolidated with allogeneic transplant) and 1 pt withdrew early from study after 14.5 mo of response. In the BR arm, 2 pts maintained responses without events; both had consolidative therapy (1 radiation, 1 allogeneic transplant). Baseline characteristics of pts with confirmed responses in the pola + BR arm are shown in the Table. In the Ph Ib cohort, 3/6 pts were confirmed responders: 1 pt progressed at 45 mo, 2 pts had ongoing responses of 29+ and 44+ mo, respectively.
Conclusions: No new safety signals were identified with longer follow-up. PN events were low grade, manageable and mostly reversible. Adding pola to BR did not increase risk of second malignancies. While median time-to-event endpoints have not changed significantly since the last data cut, longer follow-up demonstrates notable durability of response in a proportion of pts treated with pola + BR, many of whom had refractory disease and received multiple lines of prior therapy. Pola + BR represents a promising new treatment for pts with transplant-ineligible R/R DLBCL.
Sehn:Kite Pharma: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Janssen-Ortho: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Verastem: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Apobiologix: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Janssen-Ortho: Honoraria; TG Therapeutics: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Kite Pharma: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Acerta: Consultancy, Honoraria. Matasar:Bayer: Other: Travel, accommodation, expenses; Janssen: Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Honoraria, Research Funding; Daiichi Sankyo: Consultancy; Seattle Genetics: Consultancy, Honoraria, Other: Travel, accomodation, expenses, Research Funding; Rocket Medical: Consultancy, Research Funding; Teva: Consultancy; Juno Therapeutics: Consultancy; Merck: Consultancy, Equity Ownership; Roche: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Bayer: Consultancy, Honoraria, Other. Flowers:BeiGene: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Karyopharm: Consultancy; Optimum Rx: Consultancy; AstraZeneca: Consultancy; Pharmacyclics/Janssen: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Gilead: Consultancy, Research Funding; Spectrum: Consultancy; TG Therapeutics: Research Funding; Eastern Cooperative Oncology Group: Research Funding; V Foundation: Research Funding; Acerta: Research Funding; Burroughs Wellcome Fund: Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Millenium/Takeda: Research Funding; National Cancer Institute: Research Funding; Bayer: Consultancy. Kamdar:Seattle Genetics: Speakers Bureau; Genentech: Consultancy; AstraZeneca: Consultancy; Pharmacyclics: Consultancy. McMillan:Pfizer: Honoraria, Research Funding; MSD: Honoraria; BMS: Honoraria; Celgene: Honoraria, Speakers Bureau; Sandoz: Honoraria; Gilead: Honoraria; Novartis: Honoraria; F. Hoffmann-La Roche Ltd: Honoraria, Speakers Bureau. Hertzberg:Pfizer: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Assouline:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau. Kim:Novartis: Consultancy; Sanofi: Consultancy; AstraZeneca: Consultancy, Research Funding; Takeda: Consultancy; Bayer: Consultancy. Kim:F. Hoffmann-La Roche Ltd: Research Funding; Celltrion: Research Funding; Novartis: Research Funding; Donga: Research Funding; Kyowa-Kirin: Research Funding; Novartis: Research Funding; J + J: Research Funding. Ozcan:F. Hoffmann-La Roche Ltd: Other: Travel, Research Funding; Takeda: Honoraria, Research Funding; Amgen: Honoraria; Jazz: Other: Travel; Sanofi: Other: Travel; BMS: Other: Travel; MSD: Research Funding; AbbVie: Research Funding; Novartis: Research Funding; Abdi Ibrahim: Other: Travel; Janssen: Research Funding, Travel; Celgene: Research Funding; Bayer: Research Funding; Archigen: Research Funding. Croft:Genentech, Inc.: Employment. Hirata:F. Hoffmann-La Roche Ltd: Equity Ownership; Genentech, Inc.: Employment. Cheng:F. Hoffmann-La Roche Ltd: Employment. Ku:Genentech, Inc.: Employment. Herrera:Adaptive Biotechnologies: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; AstraZeneca: Research Funding; Merck: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; Pharmacyclics: Research Funding; Immune Design: Research Funding; Kite Pharma: Consultancy, Research Funding.
GO29365 (pola + BR vs BR in relapsed/refractory DLBCL). Rituximab (Rituxan) is approved for use in relapsed/refractory low-grade NHL and in previously untreated DLBCL with CHOP, but is not approved for use in relapsed/refractory DLBCL. Bendamustine (Levact) is approved for use in relapsed indolent B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Polatuzumab vedotin (Polivy) is approved for use in third-line or later treatment of R/R DLBCL in the USA.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal