Introduction: Despite therapeutic advances, there remains a considerable need for novel therapies for B-cell lymphomas. Although a high proportion of patients (pts) show response to initial therapy, many fail to achieve durable remissions and experience recurrent disease. Agents that target molecular pathways associated with the development and progression of lymphoma are likely to be highly effective and are desirable. The p110δ isoform of the PI3K enzyme is mainly expressed in lymphocytes and has been an attractive therapeutic target, with several PI3Kδ inhibitors demonstrating meaningful efficacy in B-cell lymphomas. Targeting the p110β isoform may further overcome tumor growth and escape mechanisms by mitigating the upregulation of the PI3K/AKT pathway, particularly in PTEN-deficient lymphomas. KA2237 is an oral, potent and selective inhibitor of the PI3K β and δ isoforms. The aim of this first in human, phase I, open-label, single arm study (NCT02679196) was to investigate the safety, tolerability, pharmacokinetic properties and pharmacodynamic effects of KA2237, in order to determine the maximum tolerated dose based on dose limiting toxicity and assess preliminary anti-tumor activity in pts with R/R B-cell lymphoma.
Methods: Pts ≥ 18 years (yrs) of age, ECOG ≤ 2, with B-cell lymphoma R/R or intolerant of established therapies (including rituximab) were enrolled using a 3+3 dose escalation (50-400mg) design. KA2237 was given orally on a once daily continuous schedule until progression or unacceptable toxicity. Anti-tumor activity was evaluated by computed tomography and, when available, integrating 18F-FDG positron emission tomography response assessment, at 8, 16 and 24 weeks. Response was assessed according to Lugano 2014 criteria. Pts received PJP prophylaxis.
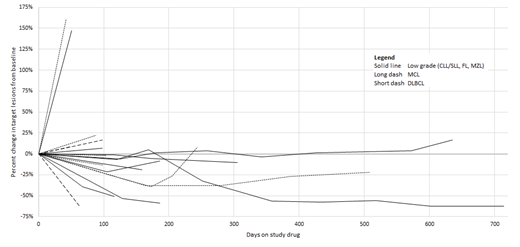
Results: 21 pts with B-cell lymphoma were enrolled (8 DLBCL [diffuse large B-cell], 5 FL [follicular], 3 MCL [mantle cell], 3 CLL/SLL [chronic lymphocytic leukemia/small lymphocytic lymphoma], 1 MZL [marginal zone], 1 WM [Waldenstrom]). Pts received KA2237 at 4 dose levels: 50mg (n=6), 100mg (n=3), 200mg (n=7) and 400mg (n=5) daily; 21 pts were evaluable for safety assessment. Pharmacokinetic profiles were favorable with mean plasma half-life of 17-33 hours, compatible with once daily oral dosing. Median age was 69 yrs (range 48-84) with 76% males; median number of prior therapies was 3 (range 1-6). Median follow up duration was 8.5 months (range 6.9-24.6). Median duration of drug exposure was 82 days (range 10-714 days). 86% of pts experienced treatment-related adverse events (TRAE). 43% of pts experienced a grade ≥ 3 TRAE, with rash (n=3), transaminitis (n=2) and pneumonitis (n=2) occurring in more than 1 pt. 29% discontinued treatment due to a TRAE with pneumonitis (n=2) occurring in more than 1 pt. One grade 5 TEAE (multifocal pneumonia) was observed. 19/21 pts were evaluable for response, ORR was 37% (4 CR, 3 PR). Responses were observed across lymphoma subtypes including DLBCL, FL, CLL and MCL. Responses were often durable (see Figure) and in 2 pts with DLBCL who achieved CR permitted consolidation by autologous stem cell transplantation.
Conclusions: KA2237 is an oral, once a day, selective dual inhibitor of PI3K β/δ with a manageable toxicity profile and promising single-agent clinical activity in heavily pretreated R/R B-cell lymphoma. The recommended phase II dose is 200mg daily. The findings of this study support the further evaluation of KA2237.
Nastoupil:Novartis: Honoraria; Spectrum: Honoraria; Janssen: Honoraria, Research Funding; Gilead: Honoraria; Genentech, Inc.: Honoraria, Research Funding; Bayer: Honoraria; Celgene: Honoraria, Research Funding; TG Therapeutics: Honoraria, Research Funding. Neelapu:Acerta: Research Funding; Merck: Consultancy, Research Funding; Poseida: Research Funding; Unum Therapeutics: Consultancy, Research Funding; Karus: Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Incyte: Consultancy; Precision Biosciences: Consultancy; BMS: Research Funding; Allogene: Consultancy; Pfizer: Consultancy; Cellectis: Research Funding; Novartis: Consultancy; Celgene: Consultancy, Research Funding; Cell Medica: Consultancy. Fowler:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; ABBVIE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Westin:Janssen: Other: Advisory Board, Research Funding; Genentech: Other: Advisory Board, Research Funding; Juno: Other: Advisory Board; Unum: Research Funding; Kite: Other: Advisory Board, Research Funding; Novartis: Other: Advisory Board, Research Funding; Curis: Other: Advisory Board, Research Funding; Celgene: Other: Advisory Board, Research Funding; 47 Inc: Research Funding; MorphoSys: Other: Advisory Board. Wang:Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Acerta Pharma: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding; MoreHealth: Consultancy, Equity Ownership; BioInvent: Consultancy, Research Funding; Aviara: Research Funding; BeiGene: Research Funding; Loxo Oncology: Research Funding; VelosBio: Research Funding; Pulse Biosciences: Consultancy; Juno Therapeutics: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Dava Oncology: Honoraria; Kite Pharma: Consultancy, Research Funding. Beer:Karus therapeutics Ltd.: Employment. Cecil:Karus Therapeutics: Employment. Dow:Karus Therapeutics: Employment. McHale:Karus Therapeutics: Employment. Silva:Karus Therapeutics: Employment. Ward:Karus Therapeutics: Employment. Yavari:Karus Therapeutics: Employment. Shuttleworth:Karus Therapeutics: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal