Introduction: Acalabrutinib (acala) is an irreversible, second generation Bruton tyrosine kinase inhibitor (BTKi) approved by the FDA for the treatment of relapsed/refractory (R/R) mantle cell lymphoma, and is in advanced stages of clinical testing for front line (FL) and R/R CLL (Byrd NEJM, Wang NEJM). Ibrutinib (ibr) is a well-established and potent treatment for CLL; however, discontinuation due to intolerance precludes a significant number of patients (pts) in clinical practice from long-term benefit of this targeted therapy (Mato et al Blood 2016, Follows BJH 2017). Acala inhibits BTK more selectively than ibr, and while demonstrating impressive activity, may result in significantly less off-target adverse events (AEs). Although not yet FDA approved, it is being increasingly used in CLL pts with ibr-intolerance or cardiovascular or hematologic comorbidities (atrial fibrillation (AF), hypercoagulable state on long-term anticoagulation, etc.) to potentially avoid ibr-related toxicities. The increasing use of acala in clinical practice provides an opportunity to address various knowledge gaps. Therefore, we conducted a multi-institutional, retrospective analysis in CLL pts treated with acala in the real-world setting. We aimed to report and analyze the main reasons for starting acala, its safety, efficacy, outcome and sequencing.
Patients and Methods: This multi-institutional, retrospective analysis included acala-treated CLL pts at 9 US cancer centers. We analyzed and described prior treatments, dosing of acala, discontinuations, toxicities and outcomes. The primary endpoint was progression-free survival (PFS) as predicted by the Kaplan Meier method.
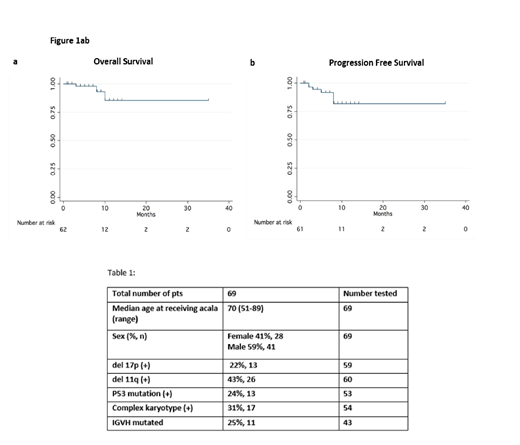
Results: 69 CLL pts treated with acala (off clinical trials) were identified. Baseline characteristics are described in Table 1. Median age was 70 years (range 51-89) and 6 (9%) and 63 (91%) pts received acala in the FL and R/R settings, respectively. Of the R/R pts, 49 (78%) had received a prior BTKi, 11 (17%) a phosphoinositide 3-kinase inhibitor (PI3Ki), 14 (22%) venetoclax, 18 (29%) bendamustine, and 17 (27%) fludarabine. Median time from diagnosis of CLL to starting acala was 79.5 months. Most common reasons for choosing acala were prior intolerance to other agents in 47 (68%), disease progression on the prior line of therapy in 19 (28%) and concern for intolerance to other therapies due to age/comorbidities in 9 (13%). Prior intolerance to ibr was the reason for starting acala in 46 (98%) of the intolerant pts; the most common AEs leading to discontinuation of ibr were rash in 10 (22%), AF/flutter in 8 (17%), arthralgia in 8 (17%), bleeding in 6 (13%), infection in 6 (13%) and fatigue in 6 (13%). Almost all pts (99%) started on acala 100 mg orally twice daily, with dose reductions required in 5 (7%). The most common toxicities on acala were fatigue in 9 (13%), infection in 9 (13%), diarrhea/colitis in 7 (10%), nausea/vomiting in 6 (9%), headache in 5 (7%), rash in 5 (7%), bleeding in 2 (3%) and AF/flutter in 1 (1%). The median time from the start of acala to an infection was 3 months. With a median follow-up of 5 months, the acala discontinuation rate was estimated to be 19% (13 pts) with median time to discontinuation of 1 month. The main reason for discontinuation was AEs in 8 pts (12%) (no consistent pattern of AEs), all in R/R pts with median time to discontinuation of 3.5 months; 2 others proceeded to CAR T-cell therapy or allogeneic transplant; 2 died of unrelated causes, 1 from progressive disease off acala. Overall response rate (ORR) was 62% (9% CR, 53% PR, number reported = 66), 50% PR in FL (3/6) and 63% (CR 5%, PR 58%, number reported = 60) in R/R pts. The differences likely reflected small numbers and short follow-up. Median PFS and overall survival were not reached (Figs 1a,b).
Conclusion: In this group of largely ibr-intolerant pts, the overall acala discontinuation rate was lower than prior reports from clinical trials, although with relatively short follow up. However, discontinuation rate due to AEs appears to be similar to clinical trial data in an ibr-intolerant pt population (Awan Blood Adv, Rogers ICML 2019). These data demonstrate the need for the results of randomized studies, in order to compare the efficacy and AE profile of acala relative to ibr and other novel agents and
Yazdy:Genentech: Research Funding; Bayer: Honoraria, Speakers Bureau; Octapharma: Consultancy; Abbvie: Consultancy. Mato:Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Celgene: Consultancy; Johnson & Johnson: Consultancy, Research Funding; TG Therapeutics: Consultancy, Other: DSMB member , Research Funding; Acerta: Consultancy; Janssen: Consultancy; Gilead: Research Funding; DTRM Biopharma: Research Funding; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; LOXO: Consultancy, Research Funding. Roeker:AbbVie: Equity Ownership; Abbott Laboratories: Equity Ownership. Ujjani:Gilead: Consultancy; Atara: Consultancy; Genentech: Honoraria; AbbVie: Honoraria, Research Funding; PCYC: Research Funding; Pharmacyclics: Honoraria; PCYC: Research Funding; Pharmacyclics: Honoraria; Astrazeneca: Consultancy. Shadman:TG Therapeutics: Research Funding; Gilead: Research Funding; Merck: Research Funding; Bigene: Research Funding; Celgene: Research Funding; Acerta: Research Funding; Emergent: Research Funding; Sunesis: Research Funding; Mustang Biopharma: Research Funding; Atara: Consultancy; Pharmacyclics: Consultancy, Research Funding; Verastem: Consultancy; AbbVIe: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; AstraZeneca: Consultancy; Sound Biologics: Consultancy; ADC Therapeutics: Consultancy. Skarbnik:Genentech: Honoraria, Speakers Bureau; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Acerta: Research Funding; Novartis: Speakers Bureau; Jazz Pharmaceuticals: Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Verastem Oncology: Honoraria, Research Funding, Speakers Bureau; Kite Pharma: Honoraria, Speakers Bureau; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; CLL Society: Consultancy, Membership on an entity's Board of Directors or advisory committees. Pagel:AstraZeneca: Consultancy; Gilead Sciences: Consultancy; Pharmacyclics: Consultancy. Patel:Sunesis: Consultancy; Pharmacyclics/Janssen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Genentech: Consultancy, Speakers Bureau. Jacobs:AstraZeneca: Speakers Bureau; Gilead: Consultancy; Genentech: Speakers Bureau; JUNO: Consultancy; TG Therapeutics: Honoraria, Research Funding; AbbVie: Consultancy, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Research Funding, Speakers Bureau. Feldman:Portola Pharma: Research Funding; Roche: Research Funding; Corvus: Research Funding; Eisai: Research Funding; Kyowa Hakko Kirin: Research Funding; Roche: Research Funding; Trillium: Research Funding; Viracta: Research Funding; Pfizer: Research Funding; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Other: Travel expenses, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Other: Travel expenses, Speakers Bureau; AbbVie: Honoraria, Other: Travel expenses, Speakers Bureau; Amgen: Research Funding; Cell Medica: Research Funding; Kite Pharma: Honoraria, Other: Travel expenses, Speakers Bureau. Goy:University of Nebraska: Research Funding; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; Hakensackumc: Research Funding; Hackensack University Medical Center, RCCA: Employment; Genentech: Other: Grants outside of the submitted work, Research Funding; Pharmacyclics/Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; Takeda: Other: Grants outside of the submitted work; Astrazenca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work; COTA: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Other: leadership role for profit healthcare company; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Coombs:Pharmacyclics: Honoraria; Loxo: Honoraria; H3 Biomedicine: Honoraria; Octopharma: Honoraria; Dedham Group: Consultancy; Cowen & Co.: Consultancy; Medscape: Honoraria; Abbvie: Consultancy; Covance: Consultancy. Lamanna:Celgene: Consultancy; Infinity/ Verastem: Research Funding; Ming: Research Funding; TG Therapeutics: Research Funding; Oncternal: Research Funding. Weiss:TG Therapeutics: Other: family member with employment and equity) . Cheson:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Research Funding; Portola: Research Funding; Kite: Research Funding; Gilead: Research Funding; Epizyme: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Symbios: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trillium: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Acalabrutinib is an irreversible, second generation Bruton tyrosine kinase inhibitor approved by the FDA for the treatment of relapsed/refractory (R/R) mantle cell lymphoma, and is in advanced stages of clinical development testing for front line (FL) and R/R CLL. Although not yet FDA approved, it is being increasingly used in CLL patients in clinical practice. This highlights the necessity to address various knowledge gaps.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal