Among patients who survive over two years post-allo-HSCT, cGVHD and subsequent cancers represent a significant source of morbidity and mortality. Long-term treatment with immunosuppressive agents and cGVHD-related immune dysregulation may promote the development of subsequent cancers. The burden of subsequent cancers has not yet been described in patients with the most severe manifestations of cGVHD, who likely represent a high-risk population.
439 patients were enrolled on the prospective NIH Chronic GVHD Natural History Study from 2004 to 2019, underwent one-week evaluation by subspecialists, and were scored in accordance with 2005 NIH criteria. Follow-up data were collected by annual survey in which patients self-reported cancer diagnoses and provided consent for confirmatory medical records. Cumulative incidence was estimated for non-melanoma skin cancer (NMSC) competing with death, relapse, or cancer other than NMSC and for cancer other than NMSC competing with death or relapse using the method of Gooley. Potential predictors of subsequent cancers including demographics, transplant characteristics, and cGVHD-related factors were assessed using Gray's test in univariable analysis and Cox proportional hazards models in multivariable analysis. Patients must have been free of post-transplant relapse, NMSC (for NMSC analyses only), and cancer other than NMSC at evaluation to be included in analysis.
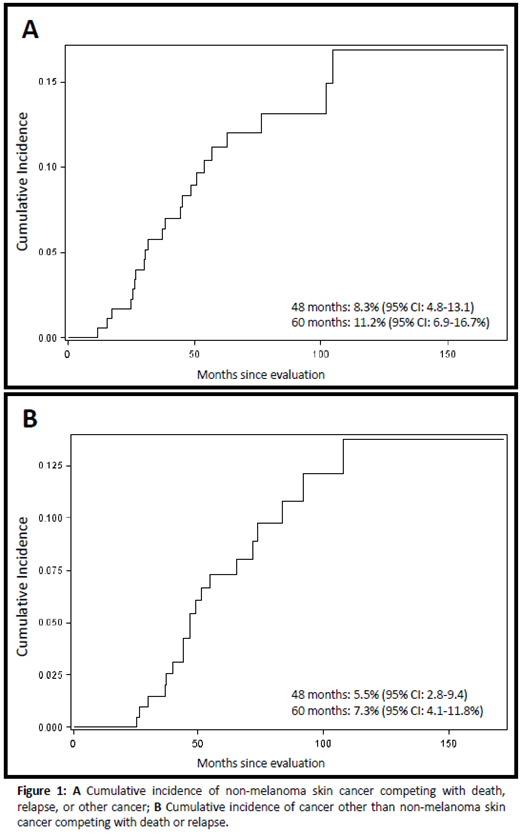
22 NMSC and 19 cancers other than NMSC were observed among 205 eligible patients, with cumulative incidences at 60 months of 11.2% (95% CI: 6.9-16.7) and 7.3% (95% CI 4.1-11.8), respectively. The most common cancers other than NMSC were oral squamous cell carcinoma and melanoma (n=6 each).
Factors associated with NMSC in univariable analysis were older age at transplant, older age at evaluation, having received sirolimus for cGVHD, having received extracorporeal photopheresis or psoralen-ultraviolet therapy for cGVHD as well as higher CRP, higher NK cell count, and greater BMI at evaluation. Only older age at transplant (HR=2.12; 95% CI: 1.35-3.31) and higher CRP (HR=9.61; 95% CI: 1.29-71.73) remained associated in the multivariable model. Factors associated with subsequent cancers other than NMSC in univariable analysis were T-cell depletion, lymphoid malignant indication for transplant, and increasing severity of oral cGVHD by NIH score. Only lymphoid malignant indication for transplant (HR=2.58; 95% CI: 1.31-5.07) remained significant in multivariable analysis.
The association of CRP with NMSC may represent an effect of cGVHD-related inflammation, with CRP previously associated with cGVHD severity. Interestingly, sirolimus was associated with increased risk of NMSC despite its purported antineoplastic effects. One study has previously reported increased risk of NMSC in allo-HSCT recipients treated with sirolimus, however, numerous studies have reported that sirolimus reduces risk of NMSC in solid organ recipients. In this study population, sirolimus was often prescribed later in patients already with refractory cGVHD and adjustment for measures of disease severity attenuated this association in multivariable modeling. The association of lymphoid indication with cancers other than NMSC may be attributable to age as patients with lymphoid malignancies were older at transplant than those with other indications. Additionally, differences in pre-transplant therapies for lymphoid vs. other indications may also contribute to this observation.
Post-transplant patients with cGVHD are at high risk of developing subsequent cancers, with higher incidence of NMSC than other cancers. This study identifies potential risk groups for subsequent cancers, highlights patients who may benefit from increased surveillance, and reiterates the need for effective cGVHD therapy to mitigate risks associated with long-term immunosuppression and immune dysfunction.
Cowen:UpToDate: Other: Royalties; Elsevier: Other: Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal