Purpose
The second Nordic lymphoma group protocol for mantle cell lymphoma (NLG-MCL 2) includes three courses of maxi-CHOP alternating with three courses of high-dose cytarabine, and followed by consolidation with high-dose therapy and autologous stem-cell transplantation (ASCT). This has been the standard of care for younger MCL patients in Sweden since year 2000. Since 2008, rituximab has been added. On a population level, it is not well described how many patients' complete treatment and undergo consolidation with ASCT and which reasons that govern this decision. Our aim was to describe selection mechanisms to ASCT, and further describe outcome for ASCT transplanted versus non-transplanted patients in different age groups.
Methods
We investigated 670 MCL patients aged 22-70 years and diagnosed 2000-2014 (among whom 416 were 22-65 years) using data from the Swedish population-based lymphoma register. Clinical, demographical, and comorbidity data was linked to the patient cohort through nationwide health care registers. Severity of comorbidity was defined according to the Charlson comorbidity index (CCI; 0,1 and 2+). Selection mechanisms were analysed by estimating odds ratios (ORs) with 95% confidence intervals (CIs) for being transplanted using univariable and multivariable logistic regression models. Patients that did not reach complete remission during induction chemotherapy and therefore not transplanted were identified and excluded when analyzing other selection mechanisms. Demographic determinants were specifically considered, including marital status (married, not married, divorced and widower) and educational level (up to 9 years, 10-12, and more than 12 years of schooling). Survival functions, by ASCT and age at diagnosis (<50, 50-59, 60-65, 66-70) were estimated using the Kaplan-Meier method. Moreover, hazard ratios (HRs) with 95% confidence intervals (CI) comparing all-cause mortality by ASCT and age were estimated using Cox regression (adjusted for calendar year of diagnosis, age at diagnosis, sex and country of birth).
Results
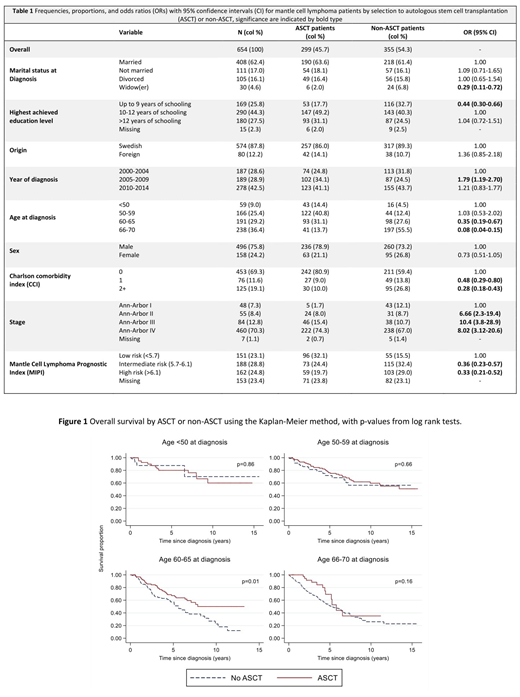
Among patients aged up to 70 years, 355 (54%) were not selected for ASCT whereas 299 (46%) were given ASCT in first remission. Among patients up to 65 years, 158/416 (38%) were not selected for ASCT. Sixteen patients did not reach complete remission after induction chemotherapy and were thus not transplanted and not further included.
Being a widow, having lower educational level, older age at diagnosis, higher comorbidity burden, diagnosed during an earlier calendar period, stage I disease and a lower mantle cell lymphoma prognostic index (MIPI) (Table 1) was in univariable analyses, associated with a reduced chance of receiving ASCT, while this was not seen for different sex, county, hospital and country of birth. In adjusted analyses of sociodemographic characteristics, being unmarried (compared to married) significantly decreased the odds of receiving ASCT (adj. OR=0.56, 95% CI: 0.34-0.94). Also, patients with any comorbidity, CCI 1 (adj. OR=0.51, 95% CI: 0.29-0.90) and severe comorbidity, CCI 2+ (adj. OR=0.41, 95% CI: 0.25-0.68) had lower chance of receiving ASCT.
For patients younger than 60 years at diagnosis MIPI and stage were generally lower in the non-transplanted group and here selection to ASCT was not associated with inferior OS (Figure 1). For patients aged 60-65 who were not selected to transplantation, overall survival was inferior compared to patients who underwent ASCT (p-value from log rank test=0.01, Figure 1). However, this association was attenuated when adjusting for potential confounders in a Cox regression (HR: 0.66, 95% CI 0.42-1.03).
Conclusion
A surprisingly high proportion of patients (more than 50%) were not treated with ASCT as part of primary treatment. Reasons for not being selected to ASCT included both medical and socioeconomic factors. Patients who were unmarried were less often transplanted. Among younger patients (<60 years), omission of ASCT seemed over-represented for "indolent" cases and no prognostic difference was seen, while for patients 60-65 years of age, omission of transplantation was associated with inferior OS. Improvements in both supportive functions, awareness for clinicians about their decisions and development of alternative, more tolerable, treatment concepts are needed for a large fraction of MCL patients and would likely improve survival.
Glimelius:Janssen Pharmaceuticals: Honoraria. Smedby:Janssen Pharmaceuticals: Other: This project was partly funded through a private-public collaboration between KI and Janssen pharmaceuticals.; Takeda: Research Funding; Celgene: Honoraria. Albertsson-Lindblad:Janssen Pharmaceuticals: Other: This study was partly funded via the public-private real world evidence collaboration between Karolinska Institutet and Janssen Pharmaceuticals (contract: 5-63/2015). Eloranta:Janssen Pharmaceuticals.: Other: project coordinator for a public-private real world evidence; Karolinska Institutet: Other: coordinator for a public-private real world evidence. Jerkeman:Janssen: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Acerta: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal