Background: Graft-versus-leukemia (GVL) responses after allogeneic hematopoietic cell transplantation (alloHCT) for AML are mediated by alloreactive donor-derived immune effector cells including T lymphocytes and natural killer (NK) cells. The function of NK cells is regulated by inhibitory and activating signals mediated through cell-surface receptors, including KIRs. Various models of NK cell alloreactivity have been associated with post-transplant outcomes, including leukemia relapse. However, these results have varied widely between different investigators employing similar models of NK cell alloreactivity. Assessment of somatic mutations in AML on post-transplant outcomes has not been investigated in the context of KIR profiles.
Methods: In this single-institution retrospective cohort study, we investigated KIR haplotypes (haplotype AA vs. Bx [associated with multiple activating KIRs]; Cooley S., et al. Blood. 113:726-732. 2009) in the context of somatic mutations. We included 34 adult patients with AML who underwent alloHCT from a matched related donor from 2006 to 2013. A targeted multi-amplicon deep NGS panel of 79 commonly mutated genes in myeloid neoplasia was performed. Post-HCT outcomes were assessed based on mutational status and KIR haplotype with Kaplan-Meier method and log-rank test.
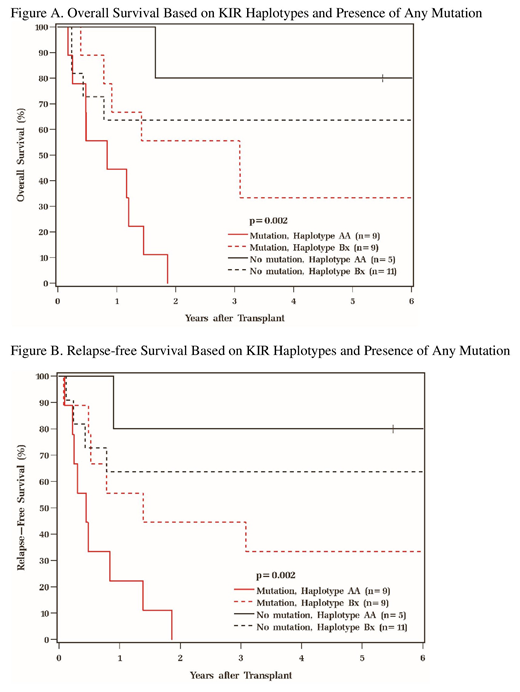
Results: Median age at transplant was 54 (range 31-73). Cytogenetic risk groups were 9% favorable, 56% intermediate, and 35% poor based on 2017 ELN classification. HCT-CI scores included 26% low, 32% intermediate, and 41% high. Disease risk group defined by ASTCT included 71% low, 26% intermediate, and 3% high. Disease status at HCT included 74% CR1 and 26% CR2. Frequencies of somatic mutations prior to HCT were: 21% DNMT3A, 18% IDH2, 9% each for STAG2 and NRAS, 6% each for ASXL1, JAK2, PHF6, RUNX1, TET2, and 3% each for CBL, FLT3, NPM1, and U2AF1. Overall, 53% of patients had at least 1 mutation: 24%, 18%, 9%, and 3% of patients had 1, 2, 3, and 4 mutations, respectively. 41% were carriers of KIR haplotype AA, and 59% were haplotype Bx. Relapse (p=0.40), relapse-free (p=0.33), and overall survival (p=0.30) between haplotypes AA and Bx were not statistically different. However, when considering somatic mutations in the context of KIR haplotypes, those with any somatic mutation (n= 18) present had inferior relapse-free (p=0.002) and overall survival (p=0.002; figures A-B) as compared to those with none. Further assessment of outcomes was then considered for those who had the following poor prognostic mutations (n=12): ASXL1, DNMT3A, FLT3, NRAS, RUNX1, and TET2. KIR haplotype AA with one or more of these mutations was associated with inferior relapse-free (p=0.05) and overall survival (p=0.008). At median follow-up of 83 (range 66-137) months, 38% were alive. Non-relapse mortality rates were 21% (9-36) at 1 year and 29% (15-39) at 3 years. The most common causes of death for all patients were relapse (48%) followed by infection (33%).
Conclusion: In presence of somatic mutations, carrying KIR haplotypes Bx was associated with better survival in AML post-alloHCT. The presence of multiple activating KIRs may also help mitigate the worse prognosis associated with some of the more deleterious somatic mutations in AML. These observations may have implications for improving patient risk stratification prior to transplant and optimizing donor selection. Future investigation with larger cohorts interrogating KIR haplotypes in the context of pre-transplant AML somatic mutations on post-transplant outcomes may further elucidate which patients may benefit most from transplant.
Nazha:Tolero, Karyopharma: Honoraria; Abbvie: Consultancy; Daiichi Sankyo: Consultancy; Novartis: Speakers Bureau; Jazz Pharmacutical: Research Funding; Incyte: Speakers Bureau; MEI: Other: Data monitoring Committee. Mukherjee:Bristol-Myers Squibb: Speakers Bureau; Projects in Knowledge: Honoraria; Celgene Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Partnership for Health Analytic Research, LLC (PHAR, LLC): Consultancy; McGraw Hill Hematology Oncology Board Review: Other: Editor; Pfizer: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Advani:Pfizer: Honoraria, Research Funding; Macrogenics: Research Funding; Glycomimetics: Consultancy, Research Funding; Kite Pharmaceuticals: Consultancy; Amgen: Research Funding; Abbvie: Research Funding. Gerds:Sierra Oncology: Research Funding; Incyte: Consultancy, Research Funding; CTI Biopharma: Consultancy, Research Funding; Imago Biosciences: Research Funding; Celgene Corporation: Consultancy, Research Funding; Roche: Research Funding; Pfizer: Consultancy. Sekeres:Celgene: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees. Majhail:Mallinckrodt: Honoraria; Atara Bio: Consultancy; Anthem, Inc.: Consultancy; Nkarta: Consultancy; Incyte: Consultancy. Maciejewski:Novartis: Consultancy; Alexion: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal